|
|
Abstract
Atopic dermatitis (AD) is a chronic inflammatory relapsing pruritic skin
disease. T-helper 2 cytokines (e.g. IL-4, IL-13) play a cardinal role in the
pathogenesis of AD. The aim of this study is to compare the effect of topical
betamethasone valerate cream 0.1% and pimecrolimus cream 1% on the profile of
IL-4 and IL-13 in patients with moderate atopic dermatitis. Twenty patients with
atopic dermatitis and 10 apparently normal volunteers were included in the
study. Ten patients were treated with topical betamethasone valerate cream
0.1%(group 1) and Ten patients with pimecrolimus cream 1 % (group 2) for 2
weeks. After 2 weeks of therapy eight cases in group 1 and six cases in group 2
showed clinical remission, up showed clinical remission . In both groups a
significant decrease in serum IL-4 and IL-13 levels after treatment was observed
, but the decrease in group1 (betamethasone valerate) was more than in group 2
(pimecrolimus cream). The IL-4 and IL-13 levels in serum after both drugs
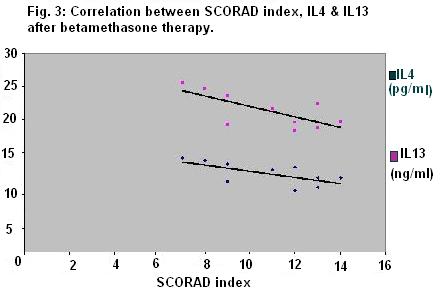
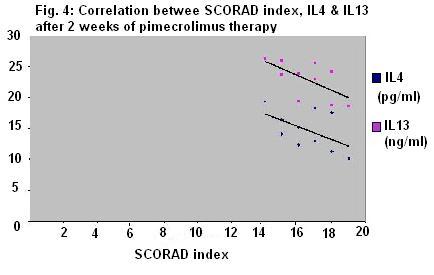
negatively correlated with the clinical severity as measured by SCORAD index.
Introduction
Atopic dermatitis is a chronic inflammatory skin disease
associated with cutaneous hyperreactivity to environmental
triggers that are innocuous to normal nonatopic
individuals[1]. The clinical phenotype that characterizes
atopic dermatitis is the product of interactions between
susceptibility genes, the environment, defective skin
barrier function, and immunologic response[2] .
Elevated levels of immunoglobulin E (IgE) have been
demonstrated in 70-80 % of patients of atopic dermatitis[3] .
The synthesis of IgE from B lymphocytes is regulated by
several cytokines derived from T lymphocytes. One of the
candidate cytokines is IL-4, which acts as switch factor for
IgE synthesis from B cells[4] IL-13 is a pleiotropic cytokine
produced by Th2-type lymphocytes and mast cells[5]. IL-13
resembles IL-4 at the amino acid level (20-25% homology),
and both share common receptor components and biological
activities[6]. IL-4, but not IL-13, induces expansion of Th2
cells and proliferation of T cells in both the mouse and
human systems[7]. IL-4 acts on both mouse and human B cells
in the same way, in that it induces class switching
including IgE, and expression of CD23 and MHC class II. In
contrast, IL-13 behaves on B cells differently in the mouse
and human systems: human IL-13 induces class switching
toward IgE and IgG4, as well as expression of CD23 and MHC
class II on B cells, whereas mouse IL-13 does not exert such
actions[8].
For almost half a century, therapy for AD has been based on
the use of emollients to alleviate dry skin, coupled with
short courses of topical corticosteroids to treat the
disease flares. Topical corticosteroids, first introduced in
the early 1950’s have been the mainstay of therapy for
atopic dermatitis for many years. This class of drugs is
generally the standard to which other therapies are
compared[9].
Topical corticosteroids are effective in the control of both
acute and chronic skin inflammation. They mediate their
anti-inflammatory effect through cytoplasmic glucocorticoid
receptors (GCR) in target cells. Upon ligand binding, the
corticosteroids / GCR complex translocates into the nucleus
where it mediates its anti-inflammatory effect via binding
transcription factors to inhibit genes encoding for
production of proinflammatory cytokines such as IL-1, IL2,
IL-4, IL-13[10]. .
Non-steroidal, topical, inflammatory-cytokine inhibitors
have been developed for the treatment of AD such as
tacrolimus ointment 0.03%- 0.1% and pimecrolimus cream 1%.
The basic mechanism of action is calcineurin inhibition[11] .
They act by binding with high affinity to the 12 kDa
macrophilin and inhibit the phosphatase activity of the
calcium-dependent phosphatase, calcineurin. In the presence
of this calcineurin inhibitor, the transcription factor,
nuclear factor of activated T cell protein (NF-ATp), is not
dephosphorylated and therefore cannot translocate into the
nucleus to activate transcription of various Th1 and Th2
cytokine genes. Pimecrolimus inhibit the activation of a
number of key effector cells involved in AD, including T
cells and mast cells[2].
Patients and methods
This study was conducted on 20 patients with moderate atopic dermatitis and 10 normal persons
as control. An oral consent was obtained from patients and controls.
The clinical severity of AD was evaluated by using the SCORAD index that
developed by the European Task Force on atopic dermatitis (1993)[12] . It defines
a score of three parameters: extent, intensity and subjective symptoms.
Extent is calculated with the rule of nines. Intensity items are erythema, edema/papulation,
oozing/crust, excoriation, lichenification and dryness of non involved skin (0
to 3 points for each item). Subjective symptoms are pruritus and sleep loss for
the last 3 days or nights (0 to 10 points for each item).
The final score is then calculated according to the following equation: A/5 + 7B + C, where A
represents extent, B represents intensity and C represents subjective symptoms.
It is considered mild moderate and severe AD forms in which SCORAD index was
less than 25, between 25 and 50 and more than 50, respectively.
The studied persons were classified into three groups:
Group 1:
Included 10 patients with moderate atopic dermatitis (7 males and 3 females),
their mean age was 8 +/- 6.5, the mean value of SCORAD index was 28.9+/- 1.8.
They were treated with topical betamethasone valerate 0.1% twice daily.
Group 2:
Included 10 patients with moderate atopic dermatitis (5males and 5females) their
mean age was 10.6 +/- 7.5. The mean value of SCORAD index was 32.1 +/- 2.2. They
were treated with topical Pimecrolimus cream 1% twice daily.
Group 3:
Control group composed of 10 normal, apparently healthy volunteers matching in
age and gender with patients in groups 1 and 2. None of them had any personal or
family history of atopy.
Serum samples were obtained from patients before and 2 weeks after treatment and
from controls .
Serum IL-4 level was measured using IL-4 ELISA Kit of Diaclone Research, France.
It is a solid phase, sandwich ELISA, monoclonal antibody specific for IL-4.
Serum IL-13 was determined by using a competitive enzyme immunoassay (EIA)
supplied from ACCUCYT R European patent EP6598 758 BI. The amount of IL-13
detected in each sample was compared to an IL-13 standard curve that
demonstrated an inverse relationship between optical density and cytokine
concentration.
Results
There was significant decrease in SCORAD index, IL-4, Il-13
after treatment in groups 1 and 2(table 1).
There was no significant difference in SCORAD index between groups 1 and 2
before treatment. The decrease in SCORAD index after treatment in group 1 was
significantly more than that in group 2 (table 2).
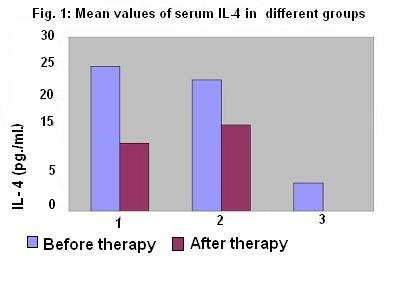
In both groups of patients, IL-4 levels were higher than in control group.
There was a statistically significant decrease in IL-4 level after treatment in
both patient groups. There was no statistically significant deference between
IL-4 level after treatment in group 1 and 2(table 3).
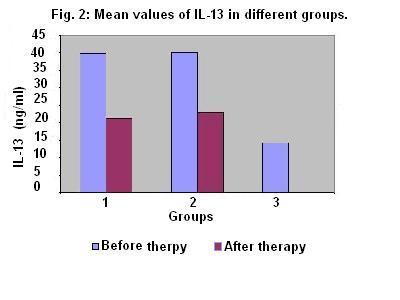
Higher IL-13 level was found in group 1and 2 than that in control group. The
decrease in IL-13 level after treatment in both patient groups showed no
statistically significant deference (table 4).
Table (1): Mean value +/- SD of age, SCORAD
index, IL-4, and IL-13
in different groups
| |
Group 1 Before treatment |
Group 1
2 weeks after treatment
|
Group 2
Before treatment
|
Group2
2 weeks after treatment
|
Control |
| Age |
8 +/- 6.5 |
|
10.6+/-7.5 |
|
9.2+/- 6.7 |
|
SCORAD |
28.9+/- 1.8 |
10.5+/- 3.5 |
32.1 +/- 2.2 |
16.5+/-2.5 |
|
| IL-4 (pg/ml) |
24.9+/-5.5 |
11.8+/2.4 |
22.6+/- 3.6 |
14.8+/-4.6 |
4.8+/-1.3 |
|
IL-13 (ng/ml) |
39.83+/-5.7 |
21.29+/-2.6 |
40.02+/-6.2 |
22.9+/-2.9 |
14.23+/-1.87 |
Table (2): Statistical comparison of SCORAD index in different
groups.
|
Comparison |
P
value |
Significance |
|
Group (1)
before treatment – Group (1) after treatment |
< 0.05 |
Significant |
| Group (2) before treatment – Group (2) after treatment |
< 0.05 |
Significant |
| Group (1) before treatment – Group (2) before treatment |
> 0.05 |
Not significant |
| Group (1) after treatment – Group (2) after treatment |
< 0.05 |
Significant |
Table (3): Statistical comparison of IL-4 in
different groups.
|
Comparison |
P
value |
Significance |
|
Group (1)
before treatment – Group (1) after treatment |
< 0.05 |
Significant |
|
Group (1)
before treatment – Control |
< 0.05 |
Significant |
|
Group (2)
before treatment – Group (2) after treatment |
< 0.05 |
Significant |
|
Group (2)
before treatment – Control |
< 0.05 |
Significant |
|
Group (1)
before treatment – Group (2) before treatment |
>0.05 |
Not significant |
|
Group (1)
after treatment – Group (1) after treatment |
>0.05 |
Not significant |
Table (4): Statistical comparison of IL-13 in
different groups.
|
Comparison |
P
value |
Significance |
|
Group (1)
before treatment – Group (1) after treatment |
< 0.05 |
Significant |
|
Group (1)
before treatment – Control |
< 0.05 |
Significant |
|
Group (2)
before treatment – Group (2) after treatment |
< 0.05 |
Significant |
|
Group (2)
before treatment – Control |
< 0.05 |
Significant |
|
Group (1)
before treatment – Group (2) before treatment |
>0.05 |
Not significant |
|
Group (1)
after treatment – Group (1) after treatment |
>0.05 |
Not significant |
Discussion
Atopic dermatitis is an inflammatory
chronically relapsing pruritic skin disease. AD is associated with elevated skin
production of Th2 cytokines and low levels of proinflammatory cytokines such as
TNF-, IFN-, and IL-1. Th2 cytokines include IL-4 and IL-13, which are known to
induce isotype switching to IgE synthesis, as well as IL-5, which plays an
important role in eosinophil development and survival [13]). Both IL-4 and IL-13
may play some important role in the development of AD [6].
Topical corticosteroids were originally introduced in 1952 as an effective means
of treating atopic dermatitis. During the past 50 years, research has focused on
strategies to optimize potency and efficacy while minimizing both local and
systemic side effects. In general, halogenated corticosteroids have been
associated with more local adverse events and a higher risk of systemic
absorption [14].
Topical corticosteroids are the standard of care to which other treatments are
compared [15].
Topical application of pimecrolimus cream 1% suppresses the production of
inflammatory cytokines associated with AD by selectively inhibiting T-cell
activation [16]. Through binding to specific receptors on T cells leading to an
increase in intracellular calcium that, in turn, causes a series of reactions
inhibiting the transcription of several genes, mainly the cytokines (IL-2, IL-4,
and IL-5) [15].
of IL-13 by peripheral blood monocytes was suppressed by use of topical
calcineurin inhibitor (tacrolimus).
In this study, 20 patients with moderate atopic dermatitis and 10 normal
volunteers were included. Ten patients treated with topical betamethasone
valerate cream 0.1% twice daily and Ten patients with pimecrolimus cream 1 %
twice daily for 2 weeks. Clinical remission were noticed in 8 cases after 2
weeks of betamethasone valerate therapy while 6 cases showed clinical remission
2 weeks after pimecrolimus treatment cream. There was significant decrease in
IL-4 and IL-13 levels in serum after treatment in both drugs, but the decrease
with betamethasone valerate was more than that with pimecrolimus cream. The IL-4
and IL-13 levels in serum after both drugs were negatively correlated with
SCORAD index that evaluate the clinical severity of AD.
Our results showed that both betamethasone valerate cream 0.1% and pimecrolimus
cream 1 % are effective in treatment of moderate AD as regard clinical
improvement (SCORAD index) and change in the cytokine pattern (IL-13 and IL-4).
Although, the decrease in the level of IL-4 and IL-13 was nearly similar in both
groups, the decrease in SCORAD index (clinical improvement) was more in the
group treated with betamethasone valerate cream 0.1%.
Although both treatments can induce remission in a relatively short time,
maintenance anti-inflammatory therapy may be required to prevent relapse. Due to
the concern about potential side effects associated with chronic use of
corticosteroids such as skin atrophy and stria, topical corticosteroids have not
been used for maintenance therapy. Nevertheless, the use of long-term
intermittent application of corticosteroids appears helpful and safe in two
randomized controlled studies [17, 18].
There are situations in which topical pimecrolimus may be advantageous over
topical corticosteroids and can be used as first line therapy Such as treatment
of the face and neck dermatitis. Pimecrolimus can be used as maintenance therapy
however, the cost benefit relation may limit its uses. Further studies for the
possible combination of topical corticosteroids and pimecrolimus are recommended
to gain effective less costly treatment and decrease possible side effects of
both drugs.
References
1-Leung D.Y. and Bieber T., Atopic dermatitis, Lancet 361:151-160, 2003.
2-Donald Y.M., Boguniewicz L.M., Michael D., et al., New insights into atopic dermatitis, J. Clin. Invest. 113:651-657, 2004.
3-Novak N., and Bieber T., Allergic and nonallergic forms of atopic diseases, J. Allergy Clin. Immunol. 112:252-262, 2003.
4- Pene J.,Rousset, F., Briere, F., et al., IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-?, J. Immunol. 141:1218-1224, 1998.
5-Zura-wski G. and de Vries J. E., Interleukin 13 and interleukin 4-like cytokine that acts on monocytes and B cells but not on T cells, Immunol. Today 15:19, 1994.
6-Takamatsu Y., Hasegawa M., Sato S., et al., IL-13 production by peripheral blood mononuclear cells from patients with atopic dermatitis, Dermatology 196:377-381, 1998.
7-Li L., Xia Y., Nguyen A., et al., Effects of Th2cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells, J. Immunol. 162:2477, 1999.
8-Umeshita-Suyama R., Sugimoto R., Akaiwa M., et al., Characterization of IL-4 and IL-13 signals dependent on the human IL-13 receptor chain 1: redundancy of requirement of tyrosine residue for STAT3 activation, International Immunology, 12:1499-1509, 2000.
9-Hoare C., Li Wan Po A. and Williams H., Systematic review of treatments for atopic eczema. Health Technol Assess 4(37):1-191, 2000.
10-Charman C.R., Morris A.D.and Williams H.C., Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol 142:931-936, 2000.
11-Bernard L.A. and Eichenfield L.F., Topical immunomodulators for atopic dermatitis, Curr. Opin. Pediatr. 14:414-418., 2002.
12- European Task Force on atopic dermatitis, Severity scoring of atopic dermatitis : the SCORAD index, Dermatology, 186:23, 1993.
13- Novak, N., and Bieber, T. Allergic and nonallergic forms of atopic diseases, J. Allergy Clin. Immunol., 112:252-262, 2003.
14-Paller A.S., Nimmagadda S., and Schachner L., Fluocinolone acetonide 0.01% in peanut oil: Therapy for childhood atopic dermatitis, even in patients who are peanut sensitive, J Am Acad. 48(4):569-577, 2003.
15- Russell J., Topical Tacrolimus: A New Therapy for Atopic Dermatitis, Am. Fam. Physician 66:1899-1903, 2002.
16- Eichenfield L. F., Lucky A. W., Boguniewicz M., et Al., Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents, J.Am.Acad. Dermatol.,46(4),495- 504, 2002.
17- Van Der Meer J.B., Glazenburg E.J., Mulder P.G., et al., The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate: The Netherlands Adult Atopic Dermatitis Study Group, Br. .J Dermatol. 140:1114-1121, 1999.
18- Hanifin J.T., Gupta AK. and Rajagopalan R., Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients., Br. J. Dermatol. 147:528-537 2002.
© 2004 Egyptian Dermatology
Online Journal
|