|
|
Abstract Background: Given that B19 infection
may present with fever, rash, non-erosive arthritis,
hepatitis, anemia, thrombocytopenia, leucopenia and positive
ANA, B19 infection may be misdiagnosed as new onset systemic
lupus erythematosus. At the same time, B19 infection and
systemic lupus erythematosus may occur simultaneously in some
patients. A casual relationship between B19 infection and
classic idiopathic systemic lupus erythematosus has not been
demonstrated yet. AIM OF WORK: This study was undertaken to
investigate the seroprevalence of parvovirus B19 in SLE
patients and to search for the different correlates of this
viremia with positive results. Subjects & Methods: Sera from
30 patients with SLE and 10 normal controls were examined for
parvovirus B19 viremia using nested polymerase chain
reaction. For each patient, clinical parameters of the
disease were also studied. Results: Parvovirus B19 DNA was
detected in 9 of the 30 patients with SLE (30 percent) while
it was not detected in any of our normal controls. Both
parvovirus-positive and negative groups of patients were on
comparably similar regimens of immunosuppression. The mean
SLAM score was higher in the parvovirus-negative patients but
the difference in SLAM score between the 2 groups was,
however, not statistically significant (P>0.05). Statistical
analysis of the clinical features and serological findings
did not yield any significant differences between the two
groups of patients. CONCLUSIONS: A cause of SLE versus an
effect of SLE relationship between the virus and the disease
is not clear. Arbitrary criteria for parvovirus- induced
SLE-like illness are suggested. IntroductionHuman parvovirus B19 (PB19) is a single-stranded DNA virus that was first discovered in 1975 by Yvonne Cossart and her colleagues [1]. Between 1975 and 1981, parvovirus B19 was a virus in search of a disease. B19 infection has been found to induce a chronic modulation of the autoimmune response [2]. Several well-defined clinical syndromes have since been attributed to parvovirus B19 infection. Some of these include transient aplastic crisis, erythema infectiosum, parvovirus B19 arthritis and several autoimmune diseases [3].
Given that B19 infection may present with fever, rash, non-erosive arthritis, hepatitis, anemia, thrombocytopenia, leucopenia and positive ANA, B19 infection may be misdiagnosed as new onset systemic lupus erythematosus (SLE) [4]. In addition, antiphospholipid antibodies, when seen in acute parvovirus B19 infection, may have the same specificity and cofactor dependence as antiphospholipid antibodies associated with systemic lupus erythematosus [5]. At the same time, B19 infection and systemic lupus erythematosus may occur simultaneously in some patients. A casual relationship between B19 infection and classic idiopathic systemic lupus erythematosus has not been demonstrated yet.
Aim of the WorkThis study was undertaken to investigate the seroprevalence of parvovirus B19 using polymerase chain reaction (PCR) in Egyptian patients with systemic lupus erythematosus and to search for the different correlates of parvovirus viremia in SLE patients with positive PCR test results.
Subjects & MethodsSera from 30 patients with SLE and ten normal controls were examined for parvovirus B19 viremia using the polymerase chain reaction.
Detection of Parvovirus B19-DNA by nested PCR:
DNA was extracted from serum by using DNA high pure PCR template preparation kit (Promega, UK) as instructed by manufacture. PCR was performed according to Zerbini et al. [6]. In brief, 7 µL of extracted DNA was added to PCR mix for a total volume of 50 µL containing 5µL of 10 x PCR buffer, 3µL of 25mM MgCl2, 2.5 U of Taq DNA polymerase (Promega, UK), 200 µM each deoxynucleotide triphosphate (Stratagen) and 300ng of each primer. After an initial denaturation step of 5 min at 95°C, the first- round PCR amplification was performed. Then 3 µL first- round product was transferred to a second 50- µL PCR mix. The second – round reaction mix contained the same constituents as the first- round mix, but 300 ng of each second primed was substituted for each first primer. The oligonucleotide primers used in the first round of amplification were
5'-CTTTAGGTATAGCCAACTGG-3´and
5´-ACACTGAGTTTACTAGTGGC-3', yielding a product of 1,112 bp. Second – round PCR was performed with primers
5'-CAAAAGCATGTGGAGTGAGG3´- and
5'- CCTTATAATGGTGCTCTGGG –3' to give a product of 104 bp. Thirty five cycles of both first and second-round amplification were performed under the following conditions after one cycle of heating at 95°C for 5 min, 95°C for 1 min, 55 °C for 1.5 min, and 72°C for 1 min, then final extension at 72°C for 7min for one cycle. Ten – microliter samples of second -round PCR products were then analyzed by electrophoresis on 2% agarose gel. Bands were visualized by ethidium bromide staining.
For each patient, the clinical parameters of disease were also studied. These included the affection of various systems by the disease (skin, kidney, CNS, joints, serous membranes), autoantibody profile (ANA and anti-DNA), hypocomplementemia, disease activity using the Systemic Lupus Activity Measure (SLAM) score and treatment.
ResultsThirty female patients suffering from SLE were included in this study. Parvovirus B19 DNA was detected in nine of the 30 patients with SLE (30 %) while it was not detected in any of our normal controls
(Figure I).
SLE patients were divided into two groups based on the presence versus absence of parvovirus B19 viremia.
Demographic features:
The two groups of patients were all females and were age and disease duration-matched (Table I)

.
Table (I): Demographic features of the two groups of patients:
Clinical and serological parameters of the disease:
Statistical analysis of the clinical features and serological findings did not yield any significant differences between the two groups of patients (Table II).
|
|
Positive cases
|
Negative cases |
|
Present |
Absent |
Present |
Absent |
Photosensitivity
|
8 |
1 |
20 |
1 |
|
Malar rash |
7 |
2 |
18 |
3 |
|
Alopecia |
5 |
4 |
12 |
9 |
|
Oral ulcers |
7 |
2 |
14 |
7 |
|
Nephritis |
3 |
6 |
6 |
15 |
|
CNS |
3 |
6 |
7 |
14 |
|
Serositis |
3 |
6 |
12 |
9 |
|
Arthritis |
9 |
0 |
21 |
0 |
|
Low C3 |
7 (77.7%) |
2 |
12 (57.2%) |
9 |
|
Low C4 |
3 |
6 |
6 |
15 |
|
ANA |
8 |
1 |
20 |
1 |
|
Anti-ds-DNA |
2 |
7 |
16 |
5 |
Table (II): Affection of various systems as well as lupus laboratory
parameters in the 2 groups of patients:
Disease activity:
The mean SLAM score was higher in the parvovirus-negative patients but the difference in SLAM score between the 2 groups was, however, not statistically significant (P>0.05) (Table III).
|
|
Positive cases
|
Negative cases |
Mean SLAM score
|
15.3 |
16.8 |
Table (III): Activity of SLE measured by the SLAM score in the two groups of SLE patients:
Treatment regimens:
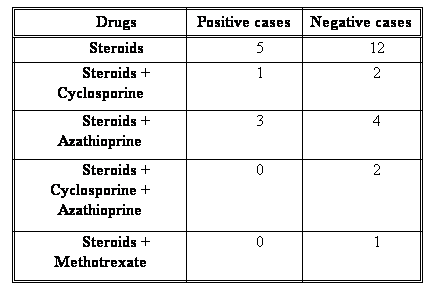
Both parvovirus-positive and negative groups of patients were on comparably similar regimens of immunosuppression (Table IV).
Table (IV): Treatment regimens received by the 2 groups of SLE patients:
 DiscussionA number of case reports describing SLE or SLE-like illness associated with human parvovirus B19 infection have been published since 1992 till today [7,
8, 9, 10,
11, 12, 13,
14, 15, 16]. However, no systematic investigation of the actual seroprevalence in epidemiologically defined SLE patients has previously been reported. Based on this plethora of case reports in the literature, acute parvovirus B19 infection may be implicated in the pathogenesis of systemic lupus erythematosus. Crowson et al. [17] prospectively followed up seven patients in whom histological and clinical signs and/or serology supported the diagnosis of a connective tissue disease (one of whom was diagnosed as SLE) in the setting of acute B19 infection. Indeed, after four years of follow up, the SLE patient was still symptomatic, B19 could be detected in skin biopsy material using PCR and serological evidence of B19 infection was still present. However, symptomatic relief followed the use of immunosuppressive and immunomo-dulatory therapy.
Human parvovirus B19 has also been suggested to trigger bouts of systemic lupus erythematosus in genetically susceptible individuals [18,
19, 20].
In our study, parvovirus viremia could be detected in 30% of the SLE population studied and in none of the normal controls (p<0.05).
On studying the characteristics of the two groups of SLE patients, those with PCR positive and those with PCR negative test results, we could not detect any statistically significant differences of note between the two groups to help delineate a cause-effect relationship. Moreover, the two groups of patients were similarly immunosuppressed by steroids and cytotoxic agents in various combinations.
We had some questions that needed answers, firstly, if parvovirus B19 infection is a cause of SLE, did it cause idiopathic SLE or it just induced a SLE-like picture that was not actually idiopathic SLE? Most of the case reports implicating parvovirus B19 as a cause of a lupus-like illness described, in addition to the lupus-like clinical features, positive serological tests for SLE, including anti-Smith antibody and also the anti-double stranded DNA which is considered by many investigators to be highly specific and committing for the diagnosis of idiopathic SLE. For this reason, we could not rely on any specific serological tests for differentiation between SLE and a parvovirus-induced lupus-like syndrome, rather we relied on disease duration and the absence of renal affection.
The disease duration of the self-limiting lupus-like disease induced by B19 infection tended to be two years or less in most of the reported cases: few weeks to a maximum of 6 months in a case series including 4 patients and a review of another 10 reported cases [8]; less than 18 months in 3 of 4 patients and persistent disease activity with repeated exacerbations in the fourth [13]; less than 1.6 years in 6 of 7 patients and 2 years in the seventh [12]; 16 weeks in an adult patient [15]; 9 months in a report of one patient [16]. Concerning our patients, the disease duration was less than two years in only four of the nine seropositive cases (44.5%) till the time at which we conducted our study, while in the remaining five patients (55.5%), it was three, four, four, five and six years respectively.
Concerning the absence of renal affection; we performed a thorough literature search for all the case reports of patients presenting with a lupus-like syndrome following parvovirus infection since the first report in 1992. We noted that the spectrum of clinical and serological features of such patients included common features and less common features. The common features included rash, fever, malaise, fatigue, arthritis, leucopenia, thrombocytopenia, and hypocomplementemia with a broad spectrum of autoantibodies. Less common ones were oral ulcers and Raynaud's phenomenon (a single report of each). As far as our knowledge, renal affection was never reported in the setting of the self-limiting lupus-like illness induced by parvovirus. Interestingly, this observation was never commented upon in the literature.
Again, concerning our cases, three of the nine seropositive patients had no renal affection, while the remaining six patients had renal affection. Interestingly, these three patients with no renal affection had disease duration of less than two years.
So, only three patients of the parvovirus positive group fulfilled the criteria of a less than two years disease duration and the absence of renal affection:
Patient 1 was 18 years old, disease duration was 1.5 years, and she presented with photosensitivity and arthritis. She had positive ANA, negative anti-DNA, complement levels were normal and SLAM score was 16.
Patient 2 was 28 years old, disease duration was 1 years, and she presented with photosensitivity, malar rash, oral ulcers and alopecia. She had positive ANA and anti-DNA, decreased C3 and SLAM score was 11.
Patient 3 was 19 years old, disease duration was 6 months, and she presented with photosensitivity, malar rash, oral ulcers, arthritis and serositis. She had positive ANA, negative anti-DNA, decreased C3 and C4 and SLAM score was 25.
It seems to us that PB19 might induce either idiopathic SLE in a person who is genetically susceptible or it might induce a SLE-like picture. The lupus illness is characterized by the classical features of SLE, which are the probability of renal disease and a disease duration of not less than two years. On the other hand, the lupus-like disease does not have usually comprise renal affection and it runs a much more benign course than that of the idiopathic type.
However, we think that until any or all of those three patients prove through follow up to have a self-limiting lupus disease, these criteria stand to be rather arbitrary and should not by any means be validated for clinical decision making. For this reason, long term follow up our patients is continuing in a trial to differentiate between the two types of SLE encountered with PB19.
Nevertheless, in order to decide that disease duration less than two years duration and the absence of renal affection should suggest a parvovirus-induced lupus-like illness, further studies are needed.
The second question that needed answering was could parvovirus B19 be an effect of immunosuppression? B19 infection in patients with SLE has been suggested to be due to a lack anti-B19 antibodies because of either the immunocompromised nature of the host or the use of immunosuppressive drugs [21]. However, according to our study, this is not feasible because both groups, the parvovirus positive and negative groups were on similar regimens of immunosuppression.
Thirdly, if parvovirus B19 infection is an effect of immunosuppression caused by SLE and/or its treatment, did it contribute to an exacerbation or heightened disease activity or not? Several studies and case reports have implicated B19 in inducing flares of SLE [18,
19]. According to our study, we do not think that parvovirus infection contributed to an increase in disease activity. On the contrary; SLAM scores tended to be higher in SLE patients who didn't have the virus rather than in those who had it.
Lastly, we questioned the following, could parvovirus B19-induced SLE benefit from antiviral therapy? Most of the reported cases were initially diagnosed as idiopathic SLE and although parvovirus B19 was documented, and sometimes at the onset, it was only suggested to trigger the lupus process. For this reason, it was never a negotiable issue to give immunosuppressives or antiviral therapy; rather all patients were started on immunosuppressive therapy.
However, we cannot guarantee that if these patients had been initially correctly diagnosed as having a parvovirus-induced lupus disease, that they would have benefited from antiviral therapy; they might have still been candidates for immunosuppressive therapy. Finally, The answer to this question lies in the actual experimentation with antiviral therapy.
Conclusions & RecommendationsParvovirus viremia was present in 30 percent of our patients.
A cause of SLE versus an effect of SLE relationship between the virus and the disease is not clear.
Based on mere observation, we suggest that a disease duration less than 2 years and the absence of renal affection might be indicators of a parvovirus-induced lupus-like illness rather than true idiopathic lupus disease though these data await confirmation in further studies.
References
1. Pattison, J. R. (1988): The discovery of human parvovirus. In Parvoviruses and Human Disease, edited by Pattison, J. R., published by Boca Raton, CRC Press, p. 1-4.
2. Vigeant, P.; Menard, H. A. and Boire, G. (1994): Chronic modulation of the autoimmune response following B19 infection. J. Rheumatol., 21 (6): 1165-7.
3. Stanley, J. and Naides, M. D. (1998): Rheumatic manifestations of parvovirus B19 infection. Rhem. Dis. Clin. N. Am., 24 (2): 375-401.
4. Fawaz-Estrup, F. (1996): Human parvovirus infection: Rheumatic manifestations, angioedema, C1 esterase inhibitor deficiency, ANA positivity and possible onset of systemic lupus erythematosus. J. Rheumatol., 23: 1180-1185.
5. Loizou, S.; Cazabon, J. K.; Walport M. J. (1997): Similarities of specificity and cofactor dependence in serum antiphospholipid antibodies from patients with human parvovirus B19 infection and from those with systemic lupus erythematosus. Arthritis Rheum., 40: 103-108.
6. Zerbini, M.; Musiani, M.; Gentilomi, G.; Venturoli, S.; Gallmella, G. and Morandi, R. (1996): Comparative evaluation of virological and serological methods in prenatal diagnosis of parvovirus B19 fetal hydrops. J. Clinic. Mycrobiol., 34: 603-608.
7. Cope, A. P.; Jones, A.; Brozovic, M.; Shafi, M. S. and Maini, R. N. (1992): Possible induction of systemic lupus erythematosus by human parvovirus. Ann. Rheum. Dis., 51 (6): 803-4.
8. Nesher, G.; Osborn, T. G. and Moor, T. L. (1995): Parvovirus infection mimicking systemic lupus erythematosus. Semin. Arthritis Rheum., 24 (5): 297-303.
9. Banno, S.; Matsumoto, Y.; Sugiura, Y. and Ueda, R. (1997): Human parvovirus B19 infection mimicking systemic lupus erythematosus: case report. Ryumachi., 37 (4): 581-6.
10. Roblot, P.; Roblot, F.; Ramassamy, A. and Becq-Giraudon, B. (1997): Lupus syndrome after parvovirus B19 infection., 64 (12): 849-5.
11. Tanaka, A.; Sugawara, A.; Sawai, K. and Kuwahara, T. (1998): Human parvovirus B19 infection resembling systemic lupus erythematosus. Intern. Med., 37 (8): 708-10.
12. Moore, T. L.; Bandlamudi, R.; Alam, S. M. and Nesher, G. (1999): Parvovirus infection mimicking systemic lupus erythematosus in a pedriatic population. Semin. Arthritis Rheum., 28 (5): 314-8.
13. Trapani, S.; Ermini, M. and Falcini, F. (1999): Human parvovirus B19 infection: its relation with systemic lupus erythematosus. Semin. Arthritis Rheum., 28 (5): 319-25.
14. Narvaez Garcia, F. J.; Domingo-Domenech, E.; Castro-Bohorquez, F. J.; Biosca, M.; Garcia-Quintana, A.; Perez-Vega, C. and Vilaseca-Momplet, J. (2001): Lupus-like presentation of parvovirus B19 infection. Am. J. Med., 111 (7): 573-5.
15. Negro, A.; Regolisti, G.; Perazzoli, F.; Coghi, P.; Tumiati, B. and Rossi, E. (2001): Human parvovirus B19 infection mimicking systemic lupus erythematosus in an adult patient., 16 (2): 125-7.
16. Kalt, M. and Gertner, E. (2001): Antibodies to beta 2-glycoprotein I and cardiolipin with symptoms suggestive of systemic lupus erythematosus in parvovirus B19 infection. J. Rheumatol., 28 (10): 2335-6.
17. Crowson, A. N.; Margo, C. M. and Dawood, M. R. (2000): A causal role for parvovirus B19 infection in adult dermatomyositis and other autoimmune syndromes. J. Cutan. Pathol., 27 (10): 505-15.
18. Hemauer, A.; Beckenlehner, K.; Wolf, H.; Lang, B. and Modrow, S. (1999): Acute parvovirus B19 infection in connection with a flare of systemic lupus erythematosus in a female patient. J. Clin. Virol., 14 (1): 73-7.
19. Langgartner, J.; Andus, T.; Hemauer, A.; Scholmerich, J. and Lang, B. (1999): Imitation of an acute exacerbation of established systemic lupus erythematosus caused by parvovirus B19 infection. Dtsch. Med. Wochenschr., 124 (28-29): 859-62.
20. Diaz, F.; Collazos, J.; Mendoza, F.; De La Viuda, J. M.; Urkijo, J. and Flores, M. (2002): Systemic lupus erythematosus associated with acute parvovirus B19 infection. Clin. Microbiol. Infect., 8 (2): 115-7.
21. Hsu, T. C. and Tsay, G. J. (2001): Human parvovirus B19 infection in patients with systemic lupus erythematosus. Rheumatology (Oxford), 40 (2): 152-7.
© 2006 Egyptian Dermatology
Online Journal
|


