|
|
Abstract
BackgroundCauses of acquired deformed or dysmorphic nails are numerous
and those affected are often very concerned about the
management of their unsightly nail problem. Correct diagnosis
is needed in order to get proper management. Nail biopsy
could be of value in order to find out or confirm the actual
diagnosis.
Aim of The Work
Evaluation of the relevance, safety and feasibility of nail
biopsy in the management of dysmorphic nail.
Patients and Methods
Fifty patients presenting with dysmorphic nail problems were
subjected to nail 4 mm punch biopsies that were driven down
to the periosteum. Biopsies were processed and tissue
sections, stained by H & E and PAS, were histopathologically
examined. According to histopathologically aided diagnosis
patients were treated and followed up.
Results
Several different nail disorders were discovered or confirmed
by nail biopsy. Data obtained by nail biopsy were a clear cut
in making the final diagnosis and prescribing the proper
treatment.
Conclusion
Nail punch biopsy is an easy and safe
procedure that dermatologists should use in the management of
dysmorphic nail disorders.
IntroductionThere is considerable concern about the diagnosis and treatment of an unsightly nail problem. The causes of acquired deformed or dysmorphic nails are numerous but onychomycosis is considered the most common cause [1,
2].
Causes of acquired dysmorphic nails
Apart from onychomycosis several other diseases can lead to dysmorphic
nails. The list may include psoriasis, lichen planus, twenty
nail syndrome, lichen sclerosis et atrophicus , eczema,
Darier`s disease and also tumors like melanocytic nevi,
Bowen's disease, melanoma, and others.
Onychomycosis, a persistent fungal infection affecting the toenails and fingernails, can interfere with standing, walking, and exercising. Associated physical impairments can result in paresthesia, pain, discomfort, and loss of manual dexterity. Patients may also suffer from loss of self-esteem and social interaction. A definitive diagnosis is crucial for effective treatment, because many other nail disorders mimic onychomycosis [2,
3].
Different pattern of presentation can be found in onychomycotic nail: DLSO
(distal and lateral subungual onychomycosis), PSO (proximal subungual onychomycosis), SWO (superficial white onychomycosis), and TDO (total dystrophic onychomycosis) [2].
Lichen planus of the nails is a well known cause of nail dystrophy and nail biopsy is considered mandatory way to confirm diagnosis [4,
5]. Lichen planus can be only presented on nails and misdiagnosis of onychomycosis is very common [6].
Twenty nail syndrome is affection of the all toes and finger nails by lichen planus although sometimes described as a separate entity [7,
8].
Nail involvement with Lichen Sclerosis is rare, in contrast to lichen planus , yet was also reported in previous reports and again biopsy was the essential tool to get to the right final diagnosis [9].
Bowen's disease could be also a cause for a dysmorphic nail as described previously. Confirmed by longitudinal biopsy and was successfully excised from a deformed dystrophic thumb nail [10].
Life threatening tumors of the nail bed such as melanomas can be misdiagnosed as traumatic, infective or benign lesions. Nail biopsy is the only way to confirm such diagnosis [11]. Differentiation from benign nevi will also be confirmed through biopsy. The number of nevi presenting with longitudinal melanonychia exceeded that of melanoma. The diagnosis of nail matrix nevi is impossible clinically and always requires histopathologic study [12].
Onychomycosis may be confused clinically with all other causes of nail dystrophy, and it is important to confirm the diagnosis of onychomycosis with appropriate laboratory or histologic analysis [6].
In practice, due to the possible misdiagnosis, at least one positive laboratory test as simple direct
microscopy or culture is now mandatory [2,
13]. Failure in diagnosis may still occur and nail histopathology could be a real useful tool to confirm the actual diagnosis [2,
14].
Nail Biopsy
Nail biopsy is a useful procedure for the diagnosis and management of many nail conditions. A basic understanding of nail anatomy and biology is a prerequisite for a successful nail biopsy. The patient must be adequately prepared and there needs to be excellent
anesthesia and hemostasis. The type of nail biopsy depends largely on the location of the pathology in the nail unit [15].
Biopsy is not merely for diagnostic purposes. Painful conditions may be relieved by the drainage of pus with proximal hemi-avulsion, or ablation of an ingrowing toenail. Focal pathology, such as a glomus
tumor or the origin of melanonychia can be completely excised besides confirming the diagnosis. If a positive diagnostic and therapeutic attitude is taken to the nail disorder, a good outcome can be expected.
A skillfully performed adequate nail biopsy, handled and processed and interpreted properly, can be an important part of a dermatologist's armamentarium in providing excellent comprehensive patient care. [16].
Types of Biopsy
Several techniques can be used to harvest a nail biopsy. Lateral longitudinal nail biopsy is the gold standard of incisional nail biopsies. It can be obtained under local
anesthetic and requires normal biopsy instruments. [17,
18].
Nail biopsy can be done using punch probes usually 4 mm driven down to the periosteum [2].
Biopsy of the nail plate alone, or with associated nail bed and occasionally matrix, may be needed to confirm fungal infection in atypical cases.
Wedge excision is considered as a new reconstructive technique after longitudinal nail biopsy [19].
Distal nail avulsion is a possible way to obtain a nail biopsy sample specially in distal affection [2].
Regular overnight fixation of the obtained sample in 10% formalin is required but another 5 days in 3% phenol may help softening nail plate material to facilitate sectioning of specimen. Subsequently, 5 micron sections can be stained with
the required stains [2,
20].
Nail Architecture
The nail plate comprises three horizontal layers: a thin dorsal lamina, the thicker intermediate lamina and a ventral layer from the nail bed. This is not always apparent with normal light microscopy using routine stains, where the nail demonstrates a transition between flattened cells dorsally and thicker cells on the ventral aspect. At high magnification, the contents of each cell show a uniform fine granularity similar to the hair cuticle.
Nail bed consists of an epidermal part (ventral matrix) with underlying connective tissue closely apposed to the periosteum of the distal phalanx. There is no subcutaneous fat in the nail bed, although scattered dermal fat cells may be visible microscopically.
The nail bed epidermal layer (ventral matrix) is usually no more than two or three cells thick, and the transitional zone from living keratinocyte to dead ventral nail-plate cell is abrupt, occurring in the space of one horizontal cell layer; in this regard it closely resembles the
Henle's layer of the internal root sheath of the epidermis. Nail bed cells do not have any independent movement, and it is yet to be clearly demonstrated whether they are incorporated into an overlying nail plate as it grows distally. The loss of the overlying nail results in the development of a granular layer, which is otherwise present only in disease states.
Nail matrix produces the major part of the nail plate. The basal compartment of the matrix is broader than the same region in normal epithelium or in other parts of the nail unit, such as the nail bed. There is no granular layer, and cells differentiate with the expression of trichocyte 'hard' keratin as they become incorporated into the nail plate. During this process, they may retain their nuclei until more distal in the nail plate. These retained nuclei are called pertinax bodies.
The nail bed dermal collagen is mainly orientated vertically, being directly attached to the phalangeal periosteum and the epidermal basal lamina. Within the connective tissue network lie blood vessels, lymphatics, a fine network of elastic
fibers and scattered fat cells; at the distal margin, eccrine sweat glands have been seen.
There is a rich arterial blood supply to the nail bed and matrix derived from paired digital arteries, a large Palmer and small dorsal digital artery on either side. The Palmer arteries are supplied from the large superficial and deep Palmer arcades.
Histological Changes in Disease Conditions
In psoriasis, Histopathology varies according to the clinical focus of the disease. The matrix and nail bed develop a granular layer. Conversely, the hyponychium, where a granular layer is normally present, no longer has one [21]. Where there is subungual hyperkeratosis, there are mounds of parakeratotic keratinocytes beneath the nail plate. Neutrophils may be found throughout these mounds and Munro microabscesses may form. Similar features are seen in acrodermatitis continua of Hallopeau [22]. Amorphous material interpreted as glycoprotein may accumulate within the keratotic mass. Acanthosis and elongation of the rete ridges is present, with increased dilatation and tortuosity of the capillaries of the dermal papillae. Where the nail is lost, the nail bed may form a false nail of compacted hyperkeratosis [23].
In twenty-nail dystrophy, there is a granular layer in the nail bed and matrix, with marked spongiosis. The hypergranulosis is believed to reflect the disordered keratinization that causes both subungual hyperkeratosis and the poor nail-plate formation. In other forms of nail lichen planus, in addition to hypergranulosis, there is occasionally saw-toothing of the rete pattern, and colloid bodies are rarely seen.
In Darier's disease, matrix and nail-bed changes resemble the acantholysis seen in involved skin with the addition of multinucleate giant cells and epithelial hyperplasia in the nail bed. These histological features make it possible to diagnose Darier's disease when it is confined to the nail [24,
25].
The pathologic features of nail matrix nevi are similar to those of skin nevi except for their architectural pattern, which reflects the peculiar anatomy of the nail unit [12].
It is essential to prepare the patient for the procedure by a discussion on a day separate from surgery. The frequency of dressings after the procedure is determined by the nature of the procedure and the patient. An antiseptic soak is normally needed to remove the dressing and clean the wound. Infection should be treated vigorously with systemic antibiotics combined with daily antiseptic soaks. It is good practice to provide a moderately strong analgesic and to recommend taking it regularly in the first 48h. It may prove unnecessary, but the distress of unrelieved pain in the few who suffer warrants caution. Some patients need night-time sedation.
In most cases, it is wise to see the patient within 2 days of surgery to personally supervise a change of dressing, review the wound and answer any questions which have arisen.
Caution is needed in patients with relevant medical and circulatory problems. The latter are subject to poor reperfusion following the tourniquet and it may be necessary to abbreviate the procedure to ensure no inadvertent damage is done. The wound must be seen to bleed and the digit
color return before the dressing is applied in these cases, and this can take several minutes. This contrasts with the usual pace at which dressings are applied to prevent bleeding in a healthy digit.
Diabetics may have ischemia combined with immune impairment and frequently need attention to toenail problems. Paradoxically, they are cited as a group in whom cold steel surgery for nail problems is preferable to other techniques such as phenolic ablation or cryosurgery. This is because the course of healing in these wounds is predictable and often shorter than wounds produced by other therapies.
Patients and MethodsFifty patients presenting with dysmorphic nail changes of one or more of the digits (toes and/or fingers) for more than 1 month were included in this study.
All patients were non diabetic and with no peculiar previous medical complains that could be related to nail affection and all changes were not dated since birth but were acquired during/after childhood.
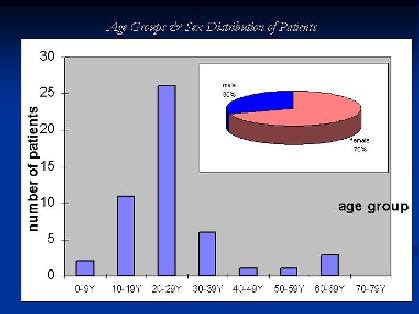
The patients mean age was 25.76 years with STD +/- 12.899, most of the cases were in their teens or twenties. Females represented 70% of patients and males 30% {Fig. 1}.
|
 |
|
Figure 1:
Patients age groups and male to female ratio. |
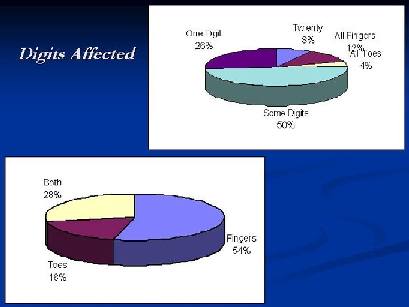
Nails of fingers were affected in 54% of patients and toes in 18% while both were affected in 28% of cases. Single digit affection was reported in 26 %, while all 20 nails affection in 8%. All fingers were affected in 12% and only all toes in 4 % and some digits in 50% of cases {Fig. 2}.
Nail changes presented in patients were: loss of luster, subungual thickening,
color changes, onycholysis, pitting, ridging and/or nail fold
edema.
All patients were subjected to regular KOH nail scrap and the procedure of nail-punch biopsy.
|
 |
|
Figure 2: Percentage of digits
affected in the study (fingers and toes) |
Nail Biopsy Procedure
The affected digit was soaked in antiseptic Betadine solution, and Xylocaine 1-2% was used as
anesthetic (No adrenaline in the anesthetic because of the risk of provoking prolonged peripheral
ischemia). Insulin syringe was used to deliver the anesthetic solution in a ring manner at the base of the affected digit.
Injection was administrated into the dorsolateral aspect of the digit at the base, with about 1-2 ml on each side of the phalanx (Digit size matter). Additional
anesthetic was given distally near to the harvesting site at the bulb of the finger/toe.
A sterile glove worn by the patient and the tip of the glove digit at the site of surgery is snipped off, and then rolled back to the base of the digit, providing a tourniquet to minimize blood flowing to the area.
Tourniquet was removed immediately after harvesting the required biopsy, making sure that its application time does not exceed 20 minutes.
The efficacy of the anesthesia was tested on skin near the harvesting site (using the tip of the used syringe). Punch probes of 3 or 4 mm diameter were used to obtain the nail specimen passing through affected nail plate to nail bed reaching to the periosteum of the distal phalanx.
Specimens were kept in 10% formalin and sent for regular histopathological preparation and H&E and PAS staining.
Sterile dressings were smoothly applied after ensuring vascular patency then hemostasis at site of procedure (after removal of the tourniquet). Analgesic (Diclofenac potassium 50mg/2/d) was prescribed in the first 2 days post-operatively.
The frequency of dressings was every 2 days with application of topical fusidic acid ointment. After one week no further dressings were needed.
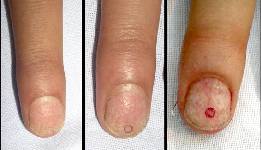
ResultsThe healing of the punch-wound in the nail was so smooth and rapid with excellent cosmetic outcome and punched scar migrated distally till disappearance completely leaving no traces of the procedure {Fig. 3}.
|
 |
|
Figure 3: Healing of the punched
area over one month duration. |
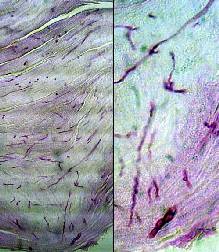
PAS staining of prepared specimens clearly demonstrated the presence of
hyphae and spores confirming fungal infection in many undiagnosed and suspected cases {Fig. 4}
|
 |
|
Figure 4: PAS positive-stained
hyphae and spores in nail plat of onychomycosis cases. |
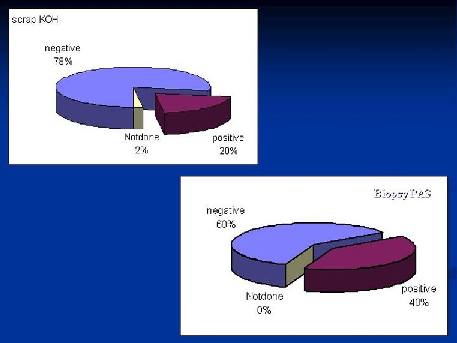
While Scarp KOH technique revealed positivity of fungal infection in only 20% of cases, pathological study of PAS stained punch-biopsy specimen revealed 40% of cases with positive stained hyphae and/or spores {Fig. 5}.
|
 |
|
Figure 5: Demonstration of hyphae
through punch biopsy procedure double that of regular
nail scraping technique. |
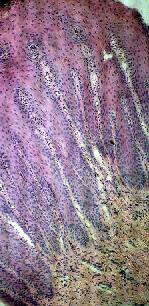
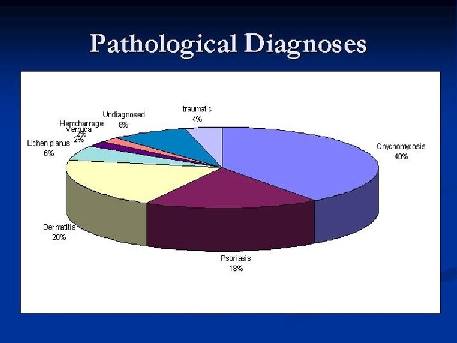
In remaining cases H&E stained specimens were studied. Twenty percent were diagnosed as chronic dermatitis where acanthosis, mild spongiosis and exocytosis were demonstrated, with glycogen trapped in keratinous plate layers that also stained positive in PAS stained slides {Fig. 6}. Eighteen percent of cases were diagnosed as psoriasis where psoriasiform pattern of epidermis was demonstrated with
Munro microabscesses in a picture similar to skin lesions with exception of presence of granular cell layer {Fig. 7}. In 6% picture similar to skin lichen planus with saw-toothing of acanthotic epidermis was demonstrated, 2% of cases were due to subungual
hemorrhage and collection of RBCs was extruded and demonstrated through biopsy specimen and the condition was relieved completely. Another 2% of cases were due to wart developing at the nail fold and extending underneath nail plate. 4% of cases were correlated to previous trauma and in 8% no clues for diagnosis could be presented and remained undiagnosed {Fig. 8}.
|
 |
 |
|
Figure 6: Acanthosis, spongiosis,
exocytosis, and trapped glycogen in epidermal keratin;
manifestation of eczema in nail bed. |
Figure 7: Psoriasiform pattern of
acanthosis with dilated vessels in elongated papillary
dermis; manifestation of psoriasis in nail bed. |
|
 |
|
Figure 8: The overall percentage of
diagnosed pathologies after nail biopsy procedure. |
Treatment was started immediately after confirming the possible diagnoses through biopsy samples.
For onychomycosis cases itraconazole was prescribed in a dosage of 200 mg twice daily for a week and repeated for 2 or 3 cycles on a monthly interval.
In cases with psoriasis dermojet intralesional triamcinolone 5% was given on 3 weeks interval. Similar dosage was given in cases with lichen planus.
For cases with eczema, suspected allergens or sensitizers such as detergents or nail polishes were strictly avoided besides prescribing topical potent steroids.
Punch biopsy was a therapeutic mode, by itself, in relieving the problem of subungual hematoma, and no further treatment modality was required.
Cryotherapy was the modality of treatment in case with ungual verruca and successfully eradicated the problem.
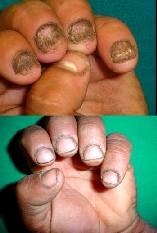
Excellent response was demonstrated in 80% of cases after only 2 months of treatment {Fig.
9,10}.
|
 |
|
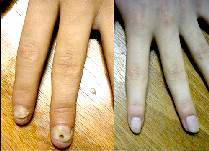
Figure 9: Onychomycosis confirmed by
nail biopsy, treated with 2 cycles of
itraconazole. |
|
 |
|
Figure 10: Nail psoriasis confirmed
by nail biopsy, treated by intralesional triamcinolone
injections.
|
|
 |
|
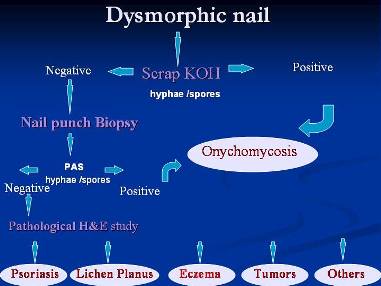
Figure 11: Suggested scheme of work
when dealing with a dysmorphic nail. |
DiscussionDysmorphic acquired nail problem is a famous bothering condition for both the patient and the doctor. The frequent inability of making accurate diagnosis usually leads to improper treatment and subsequently to a dilemma of dissatisfaction.
It was found that most of the patients seeking advice are females in their teens and twenties, this should be expected as nail dysmorphism is a cosmetic matter which will bother mainly female youngsters.
Onychomycosis used to be the most famous etiology in these situations. The use of antifungal therapy is a lengthy and costly treatment, and confirmatory data are usually required to initiate this antimycotic drug regimen.
Previously, proximal subungual onychomycosis was considered uncommon in
immunocompetent individuals [26], but now different pattern of presentation of fungal nail affection are described as mentioned before [2].
The simple presumption that dermatophytic infection starts distal while other causes of nail dystrophy start proximal is no longer applicable for empirical diagnosis and treatment.
The application of nail scraping & KOH examination or culture, could provide an objective method to confirm or deny the presence of fungal
etiology, yet failures are expected too. In our study, at least half of the onychomycosis cases were negative in KOH techniques and in previous studies similar data were obtained. Culture was also proved to be inferior in confirming the presence of fungal
etiology as reported [2].
Histopathological observation of hyphae or spores in a nail plate is a particular useful method because up to 50% of the cases may be negative on direct microscopy or culture [27].
Chronic dermatitis proved to cause a significant number of nail dysmorphism and the use of detergents, nail polishes or colorings should be properly supervised. The use of steroid topically and under occlusion helped to eliminate the dysmorphism after stopping the suspected insult in a remarkably short duration.
It is worthy to mention the positivity of PAS staining for glycogen in keratinous layers in dermatitis. This mandate proper differentiation from stained fungal hyphae or spores which we believe is easy with minimal experience.
In cases proved to be psoriasis, the use of intra-lesional triamcinolone
acetonide 5ml/cc rapidly corrected the dysmorphism but repeated injections were required is some cases when reactivation was noticed at proximal nail fold. Calcipotriol was used in only one case with good result but further trials are needed to confirm such benefit.
Patient with brown longitudinal pigmented streak at thumb nail was feared to have melanoma, yet due to the rarity of such tumor in our locality we decided to have it punch biopsied. Subungual minimal hematoma was the case and removing this collection of RBCs through the biopsy relieved the situation, confirming the relevance of nail biopsy procedure not only as a diagnostic tool but also as therapeutic modality in some instances.
The possible fear of extreme hardness of nail plate that could prevent harvesting biopsy with regular skin punch probes, has no point of relevance, but the use of new and sharp punch probes is still advised.
The healing after nail biopsy was so smooth and with excellent cosmetic outcome. The fear of mutilating the nail has no background especially when nail bed is the biopsied site. Even in a case where lesion occupied the nail matrix at
lunula, healing was also excellent and pre-biopsy longitudinal ridging, due to the pathology, improved after healing.
ConclusionDealing with dysmorphic nail problems should no longer be a dilemma and a suggested scheme of work is demonstrated in {Fig. 11}. Nail biopsy findings can enhance the accuracy of clinical diagnosis, just as skin biopsy is often crucial to the diagnosis of skin disease. Negative direct microscopy or culture can not rule out the possibility of fungal nail infection and nail biopsy remain a tool to confirm or deny such infection and so to steer the therapy to antifungal regimen or other required treatments. In addition, nail biopsy may be necessary for the early detection of a malignant tumor.
Nail biopsy is not only a diagnostic tool, but also a possible therapeutic measure in some instances. It is an easy and safe procedure that dermatologists should practice in order to confirm the suspected disease of dysmorphic nail and properly managing the patient situation.
References
1. Elweski BE, Charif MA, Daniel RCIII: Onychomycosis, in Scher RK, Daniel CRIII: eds: Nails: Therapy, Diagnosis, Surgery, WB Saunders, Philadelphia, pp 155-158, 1990.
2. Grover C, Reddy BS, Chaturvedi KU. Onychomycosis and the diagnostic significance of nail biopsy. J Dermatol. Feb;30(2):116-22, 2003.
3. Milles CL, Riley PA, Kessenich CR. Onychomycosis: diagnosis and systemic treatment. Nurse Pract. Dec;23(12):40-2, 45-8, 51, 1998.
4. Tosti A, Piraccini BM, Cambiaghi S, Jorizzo M. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. Aug;137(8):1027-32, 2001.
5. Takeuchi Y, Iwase N, Suzuki M, Tsuyuki S. Lichen planus with involvement of all twenty nails and the oral mucous membrane. J Dermatol. Feb;27(2):94-8, 2000.
6. Albert MR, Li VW, Buhac J, Dover JS, Gonzalez E. Lichen planus localized to the nails. J Cutan Med Surg. Oct;3(2):109-11, 1998 .
7. Taniguchi S, Kutsuna H, Tani Y, Kawahira K, Hamada T. Twenty-nail dystrophy (trachyonychia) caused by lichen planus in a patient with alopecia universalis and ichthyosis vulgaris. J Am Acad Dermatol. Nov;33(5 Pt 2):903-5, 1995.
8. Khandpur S, Reddy BS. An association of twenty-nail dystrophy with vitiligo. J Dermatol. Jan;28(1):38-42, 2001.
9. Ramrakha-Jones VS, Paul M, McHenry P, Burden AD. Nail dystrophy due to lichen sclerosus? Clin Exp Dermatol. Sep;26(6):507-9, 2001.
10. Mirza B, Muir JB. Bowen's disease of the nail bed. Australas J Dermatol. Nov;45(4):232-3, 2004.
11. Krige JE, Hudson DA, Johnson CA, King HS, Chetty R. S Afr J Surg. Subungual melanoma. Mar;33(1):10-4, 1995.
12. Tosti A, Baran R, Piraccini BM, Cameli N, Fanti PA. Nail matrix nevi: a clinical and histopathologic study of twenty-two patients. J Am Acad Dermatol. May;34(5 Pt 1):765-71, 1996.
13. Lawry MA, Haneke E, Strobeck K, Martin S, Zimmer B, Romano PS. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000 Sep;136(9):1112-6.
14. Gianni C, Morelli V, Cerri A, Greco C, Rossini P, Guiducci A, Braidotti P, Calcaterra R, Papini M. Usefulness of histological examination for the diagnosis of onychomycosis Dermatology.;202(4):283-8, 2001.
15. Rich A; Nail biopsy: indications and methods. Dermatol Surg. Mar;27(3):229-34, 2001.
16. Fleckman P, Omura EF. Histopathology of the nail. Adv Dermatol.;17:385-406, 2001.
17. de Berker DA. Lateral longitudinal nail biopsy. Australas J Dermatol. May;42(2):142-4, 2001.
18. Grover C, Khandpur S, Reddy BS, Chaturvedi KU. Longitudinal nail biopsy: utility in 20-nail dystrophy. Dermatol Surg. Nov;29(11):1125-9, 2003.
19. Marin Braun F, Lorea P, Ameziane L, Dury M.: Wedge excision: a new reconstructive technique after longitudinal nail biopsy. Chir Main. Oct;20(5):337-41, 2001.
20. Calvert HT, Smith MA, Wells RS. Psoriasis and the nails. Br J Dermatol. Nov;75:415-8, 1963.
21. Zaias N. Psoriasis of the nail. A clinical pathological study. Arch Dermatol; 99: 567-79, 1969.
22. Pirracini BM, Fanti PA, Morelli R, Tosti A. Hallopeau's acrodermatitis continua of the nail apparatus: a clinical and pathological study of 20 patients. Acta Derm Venereol (Stockh); 74: 65-7, 1994.
23. Samman PD. The ventral nail. Arch Dermatol; 84: 192-5, 1961.
24. Zaias N, Ackerman AB. The nail in Darier-White disease. Arch Dermatol; 107: 193-9, 1973.
25. Bingham EA, Burrows D. Darier's disease. Br J Dermatol; 111 (Suppl. 26): 88-9, 1984.
26. Piraccini BM, Morelli R, Stinchi C, Tosti A. Proximal subungual onychomycosis due to Microsporum canis. Br J Dermatol. Jan;134(1):175-7, 1996.
27. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. Apr;61(4):217-9, 1998.
© 2006 Egyptian Dermatology
Online Journal
|











