|
|
Abstract:
Background:
Different local and systemic modalities are suggested in the treatment of cutaneous leishmaniasis. But the pentavalent antimonial compounds are still considered as the first line of treatment. Regarding to increase in clinical drug resistance and adverse effects finding a more effective and safer drug is continuing.
Objectives:
To compare the effect of intralesional hypertonic sodium chloride solution and intralesional meglomine antimoniate injection in the treatment of cutaneous leishmaniasis.
Patients & Methods:
This randomized controlled clinical trial study with simple sampling was performed on 72 patients with cutaneous leishmaniasis. The patients randomly divided in two groups. One group treated with intralesional hypertonic sodium chloride solution and the other treated with intralesional meglumine antimoniate weekly 6 to 10 weeks. All patients were followed for 6 months after treatment.
Results:
After six weeks of treatment, complete improvement, partial improvement, and no response to treatment were 33.3%, 44.4% and 22.3% in meglumine antimoniate group and were 25% , 22.3% and 50% in trial group respectively. In both groups complete improvement was observed for lesions with smaller size than 2cm2. In lesions with partial improvement the treatment was repeated to 10 weeks and all patients were followed for six mounths. After six mounths ultimately cure rate was 52% in meglumine antimoniate group but 25% in hypertonic sodium chloride solution group.
Conclusion:
Injection of hypertonic sodium chloride solution has less efficacy in comparison to intralesional meglumine antimoniate in treatment of cutaneous leishmaniasis but considering the good responses in primary small lesions it can be used as a replacement therapy in some special cases including small lesions and allergic reaction to meglumine antimoniate.
Introduction:Old world cutaneous leishmaniasis usually is caused by
L. major, L. tropica, L. infantum and L. aethiopica. The reservoir of disease is
Rombomys opimus, the vector is phlebotomus and human is the accidental host[1]. After 1 to 12 week incubation period, the lesion appears as a red papule enlarging to nodule or plaque with a purple infiltrative border and central crust. Healing occurs after 6 to 12 months, but remains scar [2]. Although the disease is self limiting, for it's long duration and scarring, an effective treatment is needed. Pentavalent antimonite compounds including sodium stibogluconat (pentostam) and meglumine antimonial (Glucantime) are common treatments for cutaneous leishmaniasis [1,2], but drug resistance is increasing and the injection is painful and also has systemic complication and high cost [3,4]. Intralesional injection of Meglumine antimoniate which is a method for treatment of cutaneous leishmaniasis sometimes causes allergic reaction including prominent erythema, and edema; so that discontinuing of the treatment is needed. To find an effective, safe and Harmless drug in these circumstances, a study was carried out by using interalesional injection of hypertonic sodium chloride solution (HSCS) in Iraq and cure rate was reported 96.05% after 2-6 weeks and most lesions needed only one injection. The mechanism of hypertonic sodium chloride solution involves interference with the osmotic pressure of the cellular cytoplasm of the parasites and lesional tissues. Scarring was minimal following healing of the treated lesions [5]. In other study, cutaneous leishmaniasis lesions were treated with intralesional injection of 7% hypertonic sodium chloride plus 2% zinc sulfate and cure rate was high (94.8%) with a single injection[6]. In another study ultrastructural and Immunological features of experimental Cutaneous Leishmaniasis after treatment with intralesional hypertonic sodium chloride solution and Co2 laser rays have been evaluated and shown that IL-13 m-RNA was significantly decreased after treatment denoting that Th2 cytokine (IL-13) is important for the development of strategies to prevent the induction of pathologic processes. Also this decrease was differed according to concentration of hypertonic sodium chloride solution [7]. Considering the above studies we thought that intralesional injection of hypertonic sodium chloride solution will be used as a safe & effective treatment for Cutaneous Leishmaniasis.
Methods: This randomized controlled clinical trail study with simple sampling was done on referred population in the Skin Diseases & Leishmaniasis Research Center form an endemic foci of
L. major in Isfahan. Eligibility and exclusion criteria include following: patients with positive smear for leishman bodies, of both sexes and above 5 years old who didn't have indication for systemic therapy were included. Facial lesions or lesions on joint, sporotrichoid type, lupoid Leishmaniasis, erysipeloid type and other atypical forms of cutaneous leishmaniasis, pregnant women and patients with history of cardiovascular and renal diseases were excluded.
72 patients randomly divided in two equal groups. One group was treated with intralesional injection of meglomine antimoniate (0.5-1ml weekly) and the other group treated with hypertonic sodium chloride solution (Nacl 5%) intralesionally (0.5-1ml weekly).
Response to treatment was assessed by reepithelization and decreased induration and ulcer's size. The response to treatment is divided into three groups: complete improvement, partial improvement and no response to treatment. Complete improvement is defined as completely clinical reepithelization with no signs of induration and inflammation with negative smear for Leishman bodies at the end of the treatment period. The partial improvement is defined as the reepitheliazing lesion has become smaller but not cured. If the lesion has become larger or has not been differed it will be called as no response to treatment. The treatment was repeated for six to ten weeks and patients were followed up to 6 months.
All data were evaluated by using the chi-square (X2) test. Results:
out of 36 patients under treatment with Meglumine antimoniate most of them were under 10years old. The mean of age was 18.67%±2%. The patients had been treated for 4-6 weeks. The most common site of lesions was on the extremities(38.9%) and the least common site was the trunk (27.8%). The size range of lesions were from 0.5 to 4cm2.
In trial group out of 36 patients the most of them were under 10 years old, and mean of age was 20.52±3%. The patients had been treated for 2 to 6 weeks. The upper extremity was the most common site of lesions and the trunk was involved the least. The size range of lesions were from 0.5-4 cm2.
In treated group with Meglomine antimoniate, response to treatment was complete in 33.3%, partial in 44.4% and there was no response in 22.3% after six week. In trial group (treated with hypertonic sodium chloride solution) complete improvement, partial improvement, and no response to treatment were 25%, 22.2% and 50% respectively after six weeks. By continuing the treatment to 10 weeks in lesions with partial improvement and 6 mounths follow up ultimately complete improvement was increased to 52% in Meglomine antimoniate group but in trial group no increase in complete improvement was observed.
Table 1: Response to treatment in patients with cutaneous leishmaniasis treated with meglominee antimoniate (MG) in compared hypertonic sodium chloride solution (HSCS)
Discussion: Cutaneous leishmaniasis is a parasitic disease which is endemic in Iran.
Old world leishmaniasis is due to L. major, L. tropica, L. infantum and
L. aethiopica [1,8]. The disease is self limiting and recovery occurs after 6-12 months by remaining scar. Considering the involvement at the exposed areas e.g face and hands, there are some cosmetic, social and psychological problems in these patients, especially young women; on the other hand sometimes we encountered chronic lesions or no response to treatment. According to past studies the cure rate with intralesional injection of meglomine antimoniate was reported 40% , 50% and 65% [8,9,10], so the treatment failure is about half of cases by this method. To find a new harmless and effective treatment, regarding the effectiveness of hypertonic sodium chloride solution in past studies, this solution was compaired with intralesional meglomine antimoniate in a clinical trial.
The results in both groups were shown no significant difference between variables including age (Pv=0.67), size of lesions (Pv=0.86) sessions of treatment (Pv=0.71) and duration of disease (Pv=0.83). In evaluation of the response to treatment we observed that after 6 weeks, complete improvement were 33.2% in meglumine antimoniate group and 25% in hypertonic sodium chloride solution group (Pv=0.3) with no significant difference, but partial improvement were 44.4%, and 33.3% (Pv<0.05), and no response to treatment were 22.3% and 50% (P<0.05) in treated groups with meglumine antimoniate and hypertonic sodium chloride solution, respectively. So there is significant difference between the two groups regarding the partial improvment and no response to treatment. In lesions with partial improvement the treatment was continued to 10 weeks. All patients followed for 6 mounths and ultimately cure rate was 52% in meglumine antimoniate group and 25% in hypertonic sodium chloride solution group(P<0/05). In this study we observed that complete improvement occurred in primary small lesions (smaller than 2cm2) in both groups. Evaluation of complications was shown three sporotrichoid type, two satellite lesions and two allergic reactions as redness, edema and server pruritus around the lesions in meglumine antimoniate group. In hypertonic sodium chloride solution group, we observed three sporotichoid types but no allergic reaction. Here a question is persist which why in past studies the efficagy of HSCS were reported as 96.05% and 94.8%(HSCS plus zinc sulfat) but in this study we encountered with 25%complet improvement .It may be due to compound drugs in second but not first. As a whole, efficacy of treatment with intrealesional hypertonic sodium chloride solution is less than that of interalesional Meglumine antimoniate, but considering the good responses in small lesions in this group, the use of this method is recommended in primary small lesions with allergic reaction to Meglumine antimoniate as a replacement therapy. Also according to study which was done by soliman and Elissa and positive relation between concentration of hypertonic sodium chloride solution and decrease in the IL-13 m-RNA [7], considering the essential role for IL-13 in nonhealing lesions in some studies [11] we recommend designing a study on evaluation of increased concentration of hypertonic sodium chloride solution toward finding a new effective treatment in Cutaneous Leishmaniasis.
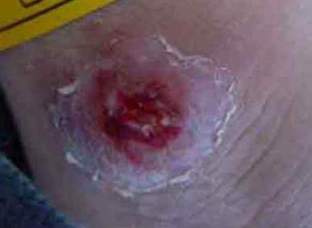
Fig 1 : A Lesion of cutaneous leishmaniasis
before treatment with intralesional injection of
hypertonic sodium chloride solution.
|
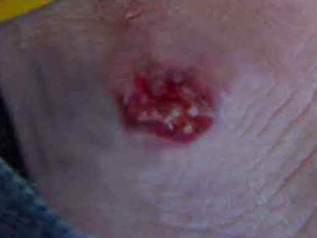
Fig 2 : Partial improvement treatment with
intralesional injection of hypertonic sodium chloride
solution.
|
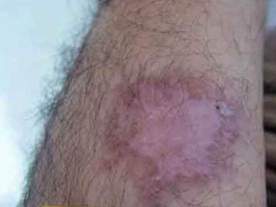
Fig 3: A Lesion of cutaneous leishmaniasis
before treatment with intralesional injection of
meglumine antimoniate. |
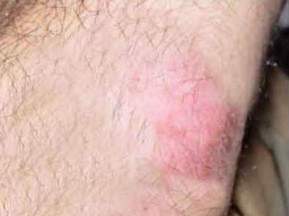
Fig 4 : Partial improvement treatment with
intralesional injection of meglumine antimoniate. |
References
1. Ardehali S, Rezaei H, Nadim A. Leishmania parasite and leishmaniasis, Tehran University publication Center, 1985. 41-65.
2. Asillian A, Cutaneous leishmaniasis & Methods of prevention treatment, Isfahan University publication Center, 1992.18-52.
3. Momeni A, Amin javaheri M, Tagvidi M, Emam Jomee M. Evaluation of Treatments & Complications of Glucantime, Journal Faize 1993, No.2. 6-10.
4. Alkawahaj AM, Larbi E. Al-Gindan y. et al. Treatment of cutaneous leishmaniasis with antimony intramuscular versus intralesional. Ann Trop med parasitol. 1997: 9: 899-905.
5. Sharquie KE. A new intralesional therapy of cutaneous leishmaniasis with hypertonic sodium chloride solution. Department of dermatology, college of medicine university of Baghdad Iraq. J., Dermatol 1995 oct; 22(10) 732-7.
6. Sharquie KE, Najim RA, Farjou IB. A comparative controlled trial of intralesionally administrated zinc sulphate, hypertonic sodium chloride and pentavalent antimony compound against acute cutaneous leishmaniasis. Clin Exp Dermatol 1997. Jul;22(4): 169
7. Eissa MM, Soliman AS, Nassar So. Ultrastructural and immunological features of experimental cutaneous leishmaniasis after treatment with intralesional hypertonic sodium, chloride and Co2 laser rays. J Egypt Soc Parasitol 2003 Apr; 33(1): 329-52.
8. Faghihi G and Tavakoli-Kia R. Treatment of cutaneous leishmaniasis with either topical paromomycin or intralesional meglumine antimoniate. Clinical and experimental Dermatology, Blackwell Publishing, 2003, 28, P. 13-16.
9. Asilian A, Sadeghinia G, Faghihi A, Momeni A, Amini Harandi A. The efficacy of treatment with intralesional meglumine antimoniate alone , compared with that of cryotherapy combined with the meglumine antimoniate or intralesional sodium stibogluconate, in the treatment of Cutaneous Leishmaniasis. Annals of Tropical Medicine & parasitology , 2003, Vol. 97, No. 5, 493-498.
10. Nilforoushzadeh MA, Reiszadeh MR, Jaffari F. Comparative effect of topical trichloroacetic acid (TCA) and intralesional glucantime in the treatment of acute cutaneous leishmaniasis, Iranian Journal of Dermatology, sixth year, 2003. No2, 36-39.
11. Alexender J, Brombacher F, Megachy HA, Mckenzie AN, Walker W, Cartor KC. An essential role for IL-13 in maintainig a non-healing response following Leishmania mexicona. Infection, Eur J Jmmunal 2002, Oct; 32(10): 2923-33.
© 2006 Egyptian Dermatology
Online Journal
|
