Abstract
Background: The effect of lasers in treating unwanted hair varies markedly with any change in pulse characteristics or in the treatment program. Many treatment parameters and regimens have been recommended and many criteria (clinical and histological) have been suggested to judge permanency of the results.
Aim of the work:
To evaluate the hair follicle stem cells and hair matrix cells under the effect of different parameter settings of hair removal lasers.
Material and methods: Ten healthy adult females (mean age 33.4 years +/- 8.6) were subjected to treatment of axillary hair by 7 different programs of hair removal laser (alexandrite or Nd:YAG lasers) at different spot sizes (7, 12, 15 & 18 mm) and at different pulse durations (5, 10, 40 and 100 milliseconds). (One program for each one of 8 quadrants of the both axillae with the 8th quadrant left untreated). Clinical evaluation was done 6 weeks and 6 months after 2 sessions. Immunohistochemical skin examination was done for cytokeratin-15 (CK-15) +v (hair follicle stem) cells and LEF-1 +ve (hair matrix) cells 6 weeks after 2 sessions.
Results:
All programs could show statistically significant reduction of hair on both follow up visits as well as significant reduction of LEF-1 score and CK-15 score as evaluated 6 weeks after 2 sessions. Significantly less results could be seen with smaller spot size of 12 mm and with 40 millisecond pulse duration. There was positive correlation between clinical results on both visits. LEF-1 score showed -ve correlation with both clinical (early and late) scores but more with the short term clinical score. CK-15 score showed strong -ve correlation with both clinical scores and positive correlation with LEF-1 score.
Conclusion: Hair follicle stem cells are important targets for the efficacy of hair removal lasers. Evaluation of hair follicle stem cells rather than hair matrix cells can represent a reliable predictor of long lasting clinical results. Introduction
In the last decade, laser and light-based technology for hair removal became one of the fastest growing procedures in modern cosmetic dermatology.[1] Light therapy can be used to destroy hair follicles by one of several mechanisms; thermal (due to local heating), mechanical (due to shock waves or violent
cavitations), or photochemical (due to generation of toxic mediators like singlet oxygen or free radicals). Hair removal has been attempted using each of these three means.[1,2,3] The thermal mechanism employed for hair removal by laser/light-based systems is based on the principle of selective photothermolysis. According to this principle, selective thermal destruction of a target will occur if sufficient energy is delivered at a wavelength well absorbed by the target within a time period less than or equal to the thermal relaxation time (TRT) of the target. Under these conditions, it is possible to selectively target the intended structure (e.g. the hair follicle) while sparing the surrounding tissues.[4] In the visible to near-infrared region, melanin is the natural chromophore for targeting hair follicles. Lasers or light sources that operate in the red or near-infrared wavelength region all lie in an optical window of the spectrum in which selective absorption by melanin is combined with deep penetration into the dermis. Therefore, deep and selective heating of the hair shaft, the hair follicle epithelium, and the heavily pigmented matrix is possible in the 600- to 1100-nm region. However, melanin in the epidermis presents a competing site for absorption. To obtain spatial confinement of thermal damage, the pulse duration should be shorter than (or equal to) the thermal relaxation time of the hair follicle but longer than that of surface epidermis (about 3 milliseconds). Thermal relaxation time of human terminal hair follicles has never been measured, but is estimated to be approximately 10-100 milliseconds, depending on follicular size. Therefore, most devices for hair removal have pulse durations in the 3-50 millisecond range. Selective cooling of the epidermis has been also applied to minimize epidermal injury.[1,2,3] As the target is melanin, the follicle must be treated in the anagen cycle. It is during the anagen phase that melanin production occurs and becomes part of the growing follicle.[5] In early anagen (in-particular), the bulb is well melanized and still fairly superficial. This represents the best timing for treatment by laser.[6] However, the bulge-activation hypothesis maintains that the bulge area of the outer root sheath near the erector pili muscle insertion contains pluripotential cells, which contribute to the new hair matrix when induced by the dermal papillae during the late telogen phase.[7] Thus, to achieve long-term hair removal, it is essential to destroy the structures that are responsible for hair growth: the bulge and bulb.[5] This has paved the way for the emergence of the so-called propagation theory and the concept of thermal damage time. Since the melanin occupies a much smaller volume compared with the follicle, heat is conducted from the melanin-rich shaft and the melanized portion of the bulb to the surrounding structures according to the laws of thermal diffusion. As it has been demonstrated that the most important targets for permanent hair removal (i.e. the stem cells) are located at the outer root sheath of the follicle at a distance from the hair shaft, and from the base of the follicle, reconsideration as to the appropriate laser parameters particularly pulse width and energy density has been recommended to allow for the propagation of the thermal damage through the entire follicular volume. This takes 3-20 times longer than the thermal relaxation time of the hair follicle. (This duration is referred to as the thermal damage time or TDT). It has been the basis of the new technology of superlong lasers as a method of more permanent hair reduction.[7,8,9] So, there seems to be some sort of controversy as regards the mechanism of action of laser and the structure(s) to target. This has been reflected on the characteristics of laser invented for the purpose of hair treatment and also on the parameters recommended for better efficacy. In fact, a lot of variables have to be addressed on treating any patient with epilatory lasers. Some are pertaining to the laser itself e.g. pulse characteristics (spot size, wave length, duration and intensity), others to the treatment protocol (e.g. timing, frequency and total number of sessions) in addition to the patient variables (e.g. color of the skin, color and thickness of the hair, other biophysical tissue characteristics, anatomic area, age , sex, and hormonal profile).[1] Putting in consideration the large number of variables to deal with and also the not yet settled nature of mechanism of action, no easy conclusions can be made and the need is always there for an objective way to assess the validity of theoretical postulations and to evaluate the efficacy of any recommended treatment regimen. The aim of the study was to evaluate the effect of variation in pulse characteristics on the clinical outcome (short and mid-term) and to assess the correlation between clinical results and the ablative effect on hair matrix and. hair follicle stem cells Material
And methods
Ten adult females have been included in the study. Excluded from the study were skin phototype V or VI, cases with recent sun tan, subjects below the age of 18 or above 45 years, photosensitive patients or those on photoactive medications, cases with previous attempts of treatment, patients suffering from major systemic or cutaneous illness, pregnant ladies, patients with clinical &/or laboratory evidence of hormonal troubles or of polycystic ovaries, cases with positive personal or family history of melanoma and those with keloidal tendency. In addition only patients with coarse, dense and black axillary hair occupying a fairly large surface area were accepted in the study. The material of the study has been subjected to 2 sessions of axillary hair treatment by laser at 6 week interval (after informed consent). In each case, four quarters have been marked on the hair bearing area of each axilla with a white indicator pencil. Identical marking in the two laser sessions as well as before clinical scoring and on taking biopsies was insured by the use of a premade template applied in fixed relation to the regional anatomic boundaries or to some already existing cutaneous landmarks (e.g. nevus or scar) and aided by digital photography under standard conditions of illumination and angles of exposure. One quarter in each case was left without treatment (as a control) while each one of the other 7 quadrants was subjected to one of the following 7 parameter settings (or programs): At 755 nm (wave length): 1) 18 mm (spot size) / 3 milliseconds (pulse duration) /12-14 j/cm2 (fluence) 2) 15 mm (spot size) / 3 milliseconds / 12-15 j/cm2 3) 15 mm (spot size) / 10 milliseconds / 12-14 j/cm2 4) 15 mm (spot size) / 40 milliseconds / 14 j/cm2 5) 12 mm (spot size) / 5 milliseconds / 14-16 j/cm2 At 1064 nm (wave length): 6) 15 mm (spot size) / 5 milliseconds / 25-30 j/cm2 7) 7 mm (spot size) / 100 milliseconds / 80-100 j/cm2 The machines used were Gentle-lase plus (Candela Corp., Wayland, MA, USA), Apogee 9300 and Apogee Elite (Cynosure, Westford, MA, USA). Cooling was done with dynamic cooling device (DCD) or continuous cold air current (Cryo-5 or Smartcool) (Cynosure, Westford, MA, USA). Protocol of treatment was as follows: no epilation for 4 weeks, no bleaching for 3 weeks, pretreatment shaving, removal of deodorants and other creams, marking, single pulse per spot, no overlap, post operative application of soothing cream plus usual post operative instructions. The areas treated were evaluated clinically (for hair density and thickness) where each criterion was given a score of 0 to 6 and the average of each of these two scores was calculated for the 10 quadrants corresponding to each program. The multiplication product of the 2 scores was designated as the overall clinical score. This has been calculated initially as well as 6 weeks and 6 months after the last (2 nd) session. A small elliptical biopsy was taken from each quadrant 6 weeks after the 2nd session. The skin specimens were immediately fixed in 10% neutral formalin and embedded in paraffin blocks for subsequent staining. The paraffin blocks were cut into 30 serial 4 um-sections and every third section was subjected to immunohistochemical staining using monoclonal antibodies against LEF-1 (as a marker of hair matrix cells)[10] (ABCAM - Cambridge - UK) or monoclonal antibodies against cytokeratin-15 (as a marker of hair follicle stem cells)[11] (ABCAM - Cambridge - UK). The procedure was done according to the manufacturer instructions. Three sections from each biopsy were also stained with
hematoxylin and eosin stain for routine histopathologic examination.
Immunohistochemistry: i. Four-micron thick tissue sections cut from the representative paraffin-embedded tissue blocks, overlaid on APES (Sigma, St. Louis, USA) coated slides, were deparaffinized (2 changes of
xylene X 5 minutes each, 1 change of acetone X 1 min) followed by rehydration in decreasing ethanol concentrations (95% ethanol X 3 mins, 70% ethanol X 3 mins, distilled water X 1 min).
ii. For staining with all the antibodies, the tissue sections were subjected to antigen unmasking by heating the sections immersed in 10 mM citrate buffer pH 6.0 (2.1 gms of anhydrous citric acid crystals dissolved in 1L of distilled water and pH adjusted to 6.0) inside a 600 watt microwave oven in full power for 35 minutes, allowed to cool to room temperature then washed briefly with 0.05 M Tris-Hcl buffer pH 7.4.
ii. Endogenous peroxidase activity was then quenched by immersing the sections in methanolic H2O2 (1 part 3% H2O2 plus 4 parts absolute methanol) for 30 minutes. After brief rinsing, the sections were placed in 0.05 M Tris-Hcl buffer pH 7.4 for 10 minutes.
iii. Sections were then overlaid with adequate amount of primary antibody diluted optimally using 0.05 M Tris-Hcl buffer pH 7.4 containing 1% bovine serum albumin (Sigma,
St .Louis, USA) followed by incubation at 40C overnight.
iv. The slides were then washed with three changes (5 mins each) of 0.05 M Tris-Hcl buffer pH 7.4 followed by incubation for 30 minutes at room temperature after application of biotinylated secondary (link) antibody in phosphate buffered saline containing carrier protein and 15 mM sodium azide (LSAB Plus Kit, DAKO, Denmark).
v. After three washings (5 mins each) in Tris-Hcl buffer, peroxidase conjugated streptavidin was applied to cover the specimens and incubated at room temperature for 30 minutes.
vi. Slides were rinsed with 3 changes of Tris-Hcl buffer for 5 mins each. Sections were then covered with substrate chromogen solution prepared freshly by dissolving 1 mg of 3,3? - diaminobenzidine tetrahydrochloride (Sigma, St. Louis, USA) in 1 ml of 0.05 M Tris-Hcl buffer pH 7.4 containing 1 ?l of hydrogen peroxide. The slides were incubated at room temperature for 5 to 10 minutes under microscopic control till the optimal development of brown colored peroxidase reaction product.
vii. After rinsing in distilled water, the sections were lightly counterstained with Harris' hematoxylin, followed by mounting with cover slips with DPX as mounting medium.
viii. Precaution was taken so that drying of tissue sections strictly did not occur at any time during the entire procedure of immunostaining. All incubations were done inside humid chambers.
ix. Controls: During each batch of staining, positive and negative controls appropriate for the particular antibody were incorporated. Primary and secondary antibodies used in the study are shown in
table (1)
|
Antigen |
1ry antibody |
2ry antibody |
|
species |
Conc. |
manufacturer |
species |
dilution |
manufacturer |
|
CK-15 |
Mouse |
01:50 |
Abcam* |
Rabbit |
0.7361111 |
Abcam |
|
LEF-1 |
Rabbit |
5 μg/ml |
Abcam |
Goat |
0.7361111 |
Abcam |
*Abcam, Cambridge, UK Table (1): Primary and secondary antibodies used in the study.
Scoring method: The stained sections were examined under the X40, X100, X200 and X400 magnification lens of light microscopy equipped with SIS image analysis computer system. For each marker, immunoreactivity was determined using a semiquantitative method in which the extent and intensity of staining was assessed on a score of 0 to 6 while the weighted score was the multiplication product of these two scores. The average of weighted score of the 10 sections examined for each biopsy was then calculated. The location and pattern of staining were also recorded. According to the degree of reduction in the average score, a final grade was given to each biopsy according to the following rule: 0-5 % reduction = 1, 5-10 % reduction = 2, 10-15 % reduction = 3, 15-20 % reduction = 4, 20-25 % reduction = 5, 25-30 % reduction = 6, 30-35 % reduction = 7, 35-40 % reduction = 8, 40-45 % reduction = 9, 45-50 % reduction = 10, 50-55 % reduction = 11, 55-60 % reduction = 12, 60-65 % reduction = 13, 65-70 % reduction = 14, 70-75% reduction = 15, 75-80 % reduction =16, 80-85 % reduction = 17, 85-90 % reduction = 18, 90-95 % reduction = 19, 95-100 % reduction = 20. The overall average grade for each set of parameters (or program) in the whole patient group was then calculated and compared with that of untreated control areas.
Statistical analysis: Comparative analysis between two groups was done through unpaired T-test using graphpad software downloaded from the website:
http://www.graphpad.com/quickcalcs/ttest1.cfm[12] Correlation between different parameters was done through graphpad software downloaded from the website:
http://calculators.stat.ucla.edu/correlation.php[13] Results
The study included 10 healthy females of skin phototype II (3 cases 30 %), type III (5 cases 50 %) and type IV (2 cases 20 %). with the age ranging between 29 and 41 years (mean 33.4 +/- 8.6) The results of clinical assessment for hair reduction (evaluated 6 weeks and 6 months after 2 sessions) are shown in
tables (2)a, b, &c. The different programs showed statistically significant reduction in the early and late clinical scores (p<0.001). The clinical results were more or less comparable for different parameters settings except for the programs number 4 and 5 which gave significantly less hair reduction at the first follow-up visit after 6 weeks, and for program 5 only at the second follow up visit (after 6 months).
|
|
|
|
|
|
6 MONTHS |
6 WEEKS |
BEFORE TREATMENT |
|
AFTER 2ND
SESSION |
AFTER 2ND
SESSION |
|
|
Overall Score |
Thickness |
Density |
Overall Score |
Thickness |
Density |
Overall |
Thickness |
Density |
|
|
score |
|
23.9+/-6.3 |
5.3 |
4.5 |
14.2 +/-2.5 |
4.9 |
2.9 |
36 |
6 |
6 |
PROG.1 |
|
26.7+/-0.4 |
5.8 |
4.6 |
15.5+/-3.0 |
5 |
3.1 |
36 |
6 |
6 |
PROG.2 |
|
20.9+/-9.1 |
5.5 |
3.8 |
16.6+/-2.7 |
5.2 |
3.2 |
36 |
6 |
6 |
PROG.3 |
|
22.1+/-7.4 |
5.4 |
4.1 |
18.8+/-4.5 |
4.7 |
4 |
36 |
6 |
6 |
PROG.4 |
|
29.0+/-2.3 |
5.8 |
5 |
22.1+/-5.5 |
4.9 |
4.5 |
36 |
6 |
6 |
PROG.5 |
|
20.3+/-9.9 |
5.2 |
3.9 |
14.6+/-1.4 |
4.7 |
3.1 |
36 |
6 |
6 |
PROG..6 |
|
20.2+/-10.2 |
5.6 |
3.6 |
16.2+/-2.6 |
4.9 |
3.3 |
36 |
6 |
6 |
PROG..7 |
|
36.0+/-0.0 |
6 |
6 |
36.0+/-0.0 |
6 |
6 |
36 |
6 |
6 |
CONTROL |
*The figures refer to the average hair score for all 10 areas treated by the
corresponding program (one area per case X 10 cases)
**At the start of treatment, all areas were supposed to be of equal maximum
density and thickness (according to the selection criteria and irrespective
to the total surface area) and were therefore given a score of 6 for
thickness and 6 for density)Table (2)a: Average clinical
score after 6 weeks and after 6 months.
|
|
Prog 1 |
Prog 2 |
Prog 3 |
Prog 4 |
Prog 5 |
Prog 6 |
Prog 7 |
control |
|
Prog 1 |
|
>0.05 |
>0.05 |
<0.05 |
<0.01 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 2 |
|
|
>0.05 |
>0.05 |
<0.01 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 3 |
|
|
|
>0.05 |
<0.05 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 4 |
|
|
|
|
>0.05 |
<0.05 |
>0.05 |
<0.001 |
|
Prog 5 |
|
|
|
|
|
<0.001 |
<0.01 |
<0.001 |
|
Prog 6 |
|
|
|
|
|
|
>0.05 |
<0.001 |
|
Prog 7 |
|
|
|
|
|
|
|
<0.001 |
(N.B. The figures shown refer to p-value for unpaired student T-test
comparing the corresponding 2 groups.) Table(2)b: Comparative analysis
of the early clinical score.
(after 6 weeks)
|
|
Prog 1 |
Prog 2 |
Prog 3 |
Prog 4 |
Prog 5 |
Prog 6 |
Prog 7 |
control |
|
Prog 1 |
|
>0.05 |
>0.05 |
>0.05 |
<0.01 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 2 |
|
|
>0.05 |
>0.05 |
<0.01 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 3 |
|
|
|
>0.05 |
<0.05 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 4 |
|
|
|
|
<0.05 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 5 |
|
|
|
|
|
<0.05 |
<0.05 |
<0.001 |
|
Prog 6 |
|
|
|
|
|
|
>0.05 |
<0.001 |
|
Prog 7 |
|
|
|
|
|
|
|
<0.001 |
(N.B. The figures shown refer to p-value for unpaired student T-test
comparing the corresponding 2 groups.) Table(2)c: Comparative analysis
of the late clinical score.
(after 24 weeks)
Histopathologically, many of the hair follicles (after 6 weeks) appeared distorted with some showing formation of dilated cystic structures (trichilemmal cyst-like) that were filled with laminated keratinous material and lined with stratified squamous epithelium. The latter was, sometimes, thinned out and atrophic and, in other instances, appeared thick and hyperplastic. Intact hair follicles were in general few and miniaturized. Assignment to one of the typical stages of hair cycle was not always possible. However, some follicles appeared to be in the early anagen phase with evidence of secondary hair germ below the lower end of late telogen follicle. Others were in the catagen stage with evidence of beginning degeneration in the lower half of hair follicle. Synchronization was not evident in most sections studied. Some follicular structures showed evidence of mild separation from the surrounding connective tissue sheath. The perifollicular area commonly showed dense homogenized collagen while in some fields remnants of mild to moderate diffuse mononuclear infiltrate could be seen throughout the dermis not only around the hair follicle. The changes were similar for all programs utilized with some case to case variations that were mostly in the degree rather than the nature of the findings observed.
(fig.1)
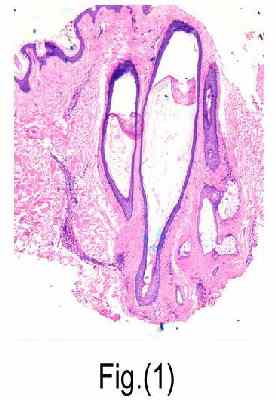 |
Fig. (1): Distorted thick follicular structures with some
forming large cysts that are filled with loose laminated keratin and
lined by thinned out stratified squamous epithelium. The perifollicular area shows dense hyalinized collagen. The overlying
epidermis is unaffected. [H&E] - [Original magnification: (X100)].
(Laser-treated skin) |
|
Immunohistochemically, all laser parameter settings could also give statistically significant reduction in LEF-1 score as a marker of hair matrix cells (p<0.001) as well as in CK-15 score as a marker of follicular stem cells (p<0.001) as evaluated 6 weeks after the last session. Again, no significant difference could be seen between different parameters except for the 755 nm ./12 mm / 5 millisecond program and 755 nm/ 15 mm/ 40 millisecond program which showed significantly less reduction in the intensity of LEF-1 immunoreactivity compared to one or more of other programs. Detailed results of the semiquantitative assessment of LEF-1
immunoreactivity 6 weeks after the second session are shown in
tables (3)a&b.
|
Score |
|
|
9.1+/- 2.6 |
Program 1 |
|
7.4+/- 1.7 |
Program 2 |
|
8.2 +/- 3.5 |
Program 3 |
|
6.1 +/- 1.6 |
Program 4 |
|
5.3+/-1.1 |
Program 5 |
|
7.8+/-2.2 |
Program 6 |
|
7.2+/- 1.5 |
Program 7 |
|
0 |
Control |
The figures refer to the average grade for the percentage of reduction in the
mean weighted score of LEF-1 +ve cells calculated for all areas treated by the
corresponding program in comparison to the control skinTable (3)a: Average
LEF-1 score after 6 weeks.
|
|
Prog 1 |
Prog 2 |
Prog 3 |
Prog 4 |
Prog 5 |
Prog 6 |
Prog 7 |
control |
|
Prog 1 |
|
>0.05 |
>0.05 |
<0.01 |
<0.001 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 2 |
|
|
>0.05 |
>0.05 |
<0.01 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 3 |
|
|
|
>0.05 |
<0.05 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 4 |
|
|
|
|
>0.05 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 5 |
|
|
|
|
|
<0.01 |
<0.01 |
<0.001 |
|
Prog 6 |
|
|
|
|
|
|
>0.05 |
<0.001 |
|
Prog 7 |
|
|
|
|
|
|
|
<0.001 |
(N.B. The figures shown refer to p-value for unpaired student T-test
comparing the corresponding 2 groups.) Table(3)b: Comparative analysis
of the LEF-1 score.
|
Score |
|
|
6.3+/-2.7 |
Program 1 |
|
5.2+/-1.9 |
Program 2 |
|
7.0+/- 2.1 |
Program 3 |
|
4.4 +/- 1.9 |
Program 4 |
|
3.3+/-1.8 |
Program 5 |
|
6.8 +/-1.6 |
Program 6 |
|
7.5 +/-3.1 |
Program 7 |
|
0 |
Control |
The figures refer to
the average grade for the percentage of reduction in the mean weighted score
of CK-15 +ve cells for all areas treated by the corresponding program in
comparison to the control skin
Table (4)a: Average CK-15 score after 6 weeks. Hair matrix cells (LEF-1 +ve cells) could not be identified in most treated sections studied indicating absence of anagen follicles, When present, they were, in
general, less intensely stained than control skin and occupied much smaller areas at the lower end of the follicles (the bulb) which -in turn- appeared to be miniaturized.
[Figs.(2)a,b and fig.(3)]
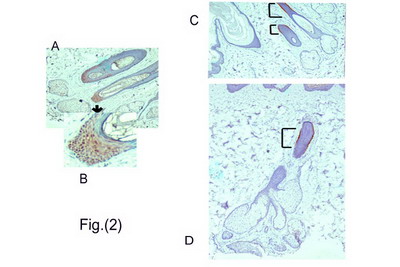
Figs.(2)a&b:
LEF-1 +ve hair matrix cells (brown nuclear staining) in the bulb
region. (Untreated skin)
Figs.(2)c&d:
Anagen follicle with CK-15+ve cells (brown) forming a thin cylinder
of basal cells in the outer root sheath of mid-follicular area
(?isthmus). (Brackets refer to CK-15 +cells.) (Untreated skin). |
|
In contrast, immunoreactivity for hair follicle stem cells could be identified in one or more sections of most of the biopsies studied but again with marked variation in the density and location of labeled cells. In control skin, they appeared as thin narrow area in the basal layer of the outer root sheath in the lower isthmus.
[Figs.(2)c,d]
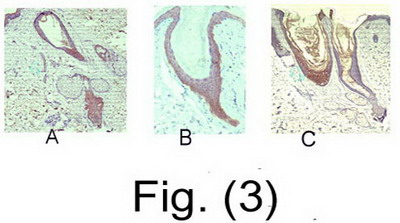
|
Figs.(3)a,b&c: LEF-1 +ve matrix cells in a cone shaped area
at the lower end of miniaturized hair follicles. (Laser-treated
skin) |
|
Histologically, the area of the so called bulge could not be identified in most biopsies examined. In the treated areas, the CK-15+ve cells showed lower intensity of immunoreactivity, occupied a smaller and thinner area around the hair follicle and were identified in only a small fraction of slides examined for each biopsy. They could be identified even around some of the distorted cystic structures though infrequently. In other areas, they appeared to occupy the secondary germ or form multiple bulge-like formations below a late telogen follicle (probably indicating entry into early anagen stage). In other areas, they were seen to occupy the isthmic region in the upper part of a catagen follicle. In areas of anagen like follicles, they appeared to be situated in the mid follicular region (supposed location of lower isthmus).
[fig.(4)]
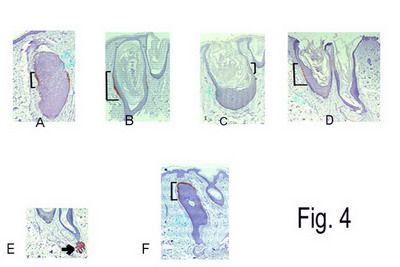
Figs.(4)a to d: Cystic follicular structures with faint
staining for CK-15+ve cells in the basal layer of the outer root
sheath of midfollicular area. (Brackets refer to CK-15 +cells.)
(Laser-treated skin)
Fig.(4)e: Morphologic bulges showing positive staining for
CK-15 (Brackets refer to CK-15 +cells.) (Laser-treated skin)
Fig.(4)f: Catagen follicle with CK-15+ cells at the lower
portion of isthmus above the degenerating lower follicle. (Brackets
refer to CK-15 +cells.) (Laser-treated skin)
|
|
Detailed results of semiquantitative assessment of CK-15 +ve cells are shown in
tables (4) a & b. The reduction was significant in all cases treated (p<0.001) but was most marked with 1064 nm (especially the super long-pulse program number 7) and again was significantly less with program 5.
|
|
Prog 1 |
Prog 2 |
Prog 3 |
Prog 4 |
Prog 5 |
Prog 6 |
Prog 7 |
control |
|
Prog 1 |
|
>0.05 |
>0.05 |
>0.05 |
<0.01 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 2 |
|
|
>0.05 |
>0.05 |
<0.05 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 3 |
|
|
|
<0.01 |
<0.001 |
>0.05 |
>0.05 |
<0.001 |
|
Prog 4 |
|
|
|
|
>0.05 |
<0.01 |
<0.05 |
<0.001 |
|
Prog 5 |
|
|
|
|
|
<0.001 |
<0.01 |
<0.001 |
|
Prog 6 |
|
|
|
|
|
|
>0.05 |
<0.001 |
|
Prog 7 |
|
|
|
|
|
|
|
<0.001 |
(N.B. The
figures shown refer to p-value for unpaired student T-test comparing the
corresponding 2 groups.) Table(4)b:
Comparative analysis of the CK-15 score.
Some degree of correlation (though not strong) could be seen between the clinical score at 6 weeks and that at 6 months. Strong negative correlation could be also seen between LEF-1 score and the early but not the late clinical score. On the other hand, both early and late clinical scores showed strong negative correlation with CK-15 score. Positive correlation could be seen between CK-15 and LEF-1 scores.
Table (5)
|
CLINICAL |
CLINICAL |
CK-15 |
LEF1 |
|
|
SCORE |
SCORE |
SCORE |
SCORE |
|
After 6 months |
After 6 weeks |
|
|
|
|
|
|
|
|
-0.4542 |
-0.9005 |
0.75992 |
|
LEF1 |
|
SCORE |
|
-0.8077 |
-0.787 |
|
|
CK-15 |
|
SCORE |
|
0.54284 |
|
|
|
CLINICAL |
|
SCORE |
|
After 6 weeks |
|
|
|
|
|
CLINICAL |
|
SCORE |
|
After 24 weeks |
(figures represent correlation coefficient)Table (5) Correlation matrix:
showing the degree of correlation between
clinical, LEF-1 and CK-15 scores.
Discussion
Several attempts have been made to assess degree of permanency in hair removal by lasers. The consensus is that lasers would induce complete but temporary hair removal (due to premature entry of hair follicle into telogen) and long term but partial reduction of the hair (due to structural damage with follicular miniaturization and perifollicular fibrosis).[1] A number of therapeutic effects have been suggested as essential prerequisites for permanent results. At first, it was suggested that involvement of the bulb is the most important effector. More recently, light has been focused on the importance of targeting stem cells.[5,14] However, there is now growing interest in the role of dermal papilla so that its involvement by laser has been suggested as essential for any long term effect on subsequent hair growth[15,16,17,18] Difficulties to assess the results of any hair treatment procedure stem from the need to wait for relatively long time to observe the clinical long term effects, the impractical necessity to take biopsies from the hair bearing areas and the inability to continue evaluation over multiple sessions on the same spot once a biopsy has been taken.[1] Most of the previous studies on hair removal by laser were meant with clinical assessment and even though the evaluation has not been carried out for a sufficient period of time.[1] Some studies, however, addressed the histopathological aspects of laser-induced changes.[19,20] To our knowledge, this is the first study to explore the effect of laser on hair follicle stem cells. Till recently, it was somewhat difficult to label hair follicle stem cells for the purpose of research studies. Earlier attempts relied upon detection of colony forming abilities of isolated cells. Others reported the use of ?1-integrin as a stem cell marker with some reservations as regards specificity and sensitivity.[21] More recently, cytokeratin-15 (an intermediate filament protein expressed by follicular stem cells and fetal -but not adult- skin cells) has been suggested as a hair follicle stem cell marker of high degree of sensitivity and specificity.[14] In our study, most treatment parameters have yielded comparable results in terms of reduction in the clinical score estimated 6 weeks and 6 months after the 2nd session except for significantly reduced efficacy of the smaller spot size of 12 mm (at both follow up visits) and of the relatively long pulse duration of 40 milliseconds (at the first follow up only). The superior results with larger spot size are understandable keeping in mind scattering of light within the tissue. The divergence of laser in a prismatic way after it collides with skin, results in marked reduction of the irradiance as the laser travels through the skin. The narrower the base of the prism at the skin surface, the greater would be the reduction in the irradiance in the depth and vice versa. In other words, larger spot sizes would achieve higher energy densities at the target level than smaller spot sizes in spite of similar energy density at the surface.[22] The apparent (or initial) decrease in the efficacy at the clinical level with moderate increase in the pulse duration (40 milliseconds) may be explained in several ways. First, the increase in duration would be accompanied by a decrease in the energy density and consequently in the peak of the target temperature. At the same time, modest increase in the duration may not be enough to achieve heat transfer to a sufficient degree. This may be attributed to the relatively large and deep nature of axillary hair follicle and consequently the long distance the heat has to travel to reach the bulge. In other words, TRT would be already long and the TDT should be even several times that of TRT.[4,7,8,9,23] However, the delayed follow-up could show results comparable to the average result indicating the ability of this relatively long pulse to achieve effective follicular damage. On the other hand, super long pulse showed somewhat enhanced efficacy although the difference in hair reduction was not statistically significant compared to some of the shorter pulse programs (5 and 10 msec). A possible explanation for insignificant difference (in contrast to what is expected according to the propagation theory) is the rather poor absorption of Nd:YAG by melanin, a fact that is actually compensated for by the poorer absorption at the skin surface and the better penetration of the 1064 nm wave length.[24,25] A more likely explanation of insignificant enhancement of results in spite of theoretically better targeting of stem cells is the presence of another reservoir for slow cycling cells. Ito et al. (2002)[26] has demonstrated the presence of the so-called secondary germ and could identify it very near to the hair bulb. This may act to replenish stem cells lying higher up in the bulge if the latter have been exposed to a strong insult. In this way, the traditional lasers with their thermolytic effect more focused on the lower portions of hair follicles rather than being dissipated over a large front would restore some credibility as opposed to the newer generation of superlong pulsed lasers. The degree of insult to hair matrix cells has been assessed 6 weeks after second session. Although there was evidence of marked structural damage (as shown by significant reduction in immunoreactivity), there was only weak negative correlation with the clinical score after 6 months. This may indicate that hair matrix cells can not represent an accurate indicator of long term laser effects as long as the hair follicle stem cells are not sufficiently affected. In other words, the hair matrix cells (which are likely to be more affected by traditional lasers) may show evidence of marked structural damage on the short run which unless paralleled by equivalent involvement of follicle stem cells would be short lasting with capability for regeneration. The strong negative correlation between the long term clinical results (after 6 months) and the immunohistochemical findings for CK-15 observed after 6 weeks may highlight the value of targeting stem cells for long term efficacy and may also provide a reliable short term and relatively rapid prognostic indicator to assess the efficacy of any newly introduced hair treatment procedure. The moderate discrepancy between the effects of laser on hair matrix versus hair stem cells may be attributed to the laser dynamics (which needs a chromophore to be absorbed) or to the difference in the cell dynamics of both cell populations. The slow cycling nature of stem cells may lend them less vulnerable to damage by laser than the rapidly cycling matrix cells.[1] So, an inadequate laser insult may be just sufficient to induce some sort of temporary functional impairment rather than a structural damage to these stem cells which probably after a long time of latency become capable again of proliferation to replenish the more acutely damaged hair matrix cells. The assumption of prolonged latency may thus explain the difference in the degree of reduction of the two cell populations on the short run in spite of the fact that the actual source of hair matrix cells are the hair follicle stem cells themselves. Apart from being a long lasting reservoir to replenish the rapid-cycling but short-lived hair matrix cells, follicular stem cells are characterized by being persistent even during the catagen and telogen stages[27], a finding that distinguishes them from hair matrix cells and adds another dimension to their importance for research purposes. While in animal studies, the stem cells have been shown to exist in a specific anatomic location (the so-called bulge area at the insertion of erector pili muscle), such anatomic landmark could not be identified in the human follicle. Instead, there is evidence in the human hair follicle that stem cells might occupy a more diffuse area along the external root sheath extending well down close to the bulb.[21] This has been also shown in our study where CK-15 +ve cells could be identified as a thin but long area along the outer root sheath near the isthmic region of the anagen follicle and high up near the epidermis in the catagen stage. In the present study, the period of 6 weeks was chosen for histochemical evaluation so as to give a chance for any possible recovery to take place at the cellular level thus increasing the probability of permanency of the observed immunohistochemical findings at least for the slow cycling stem cells. A technical problem was to distinguish between live cell and cell debris (after thermablation by laser) as the tissue section may only traverse part of the narrow immunoreactive area. This is another reason for the timing of immunohistochemical examination to ensure that only intact cells would be there to react for the corresponding marker. An important issue in interpreting the results is that evaluation of any findings should consider altered dynamics of the hair follicle after laser. This, in fact has resulted in too much confusion in the literature as regards the proper duration after which we could assess permanency. While some authors suggested evaluation after a period equivalent to the longest telogen phase of the hair follicle in the particular area, others expressed the need for a much longer period (that varied from one author to another) to give a chance for the hair follicle to recover from the laser-insult.[28] In general, the number of hairs regrowing must be stable over time greater than the duration of the complete growth cycle of hair follicles, which varies from 4 to 12 months according to body location.[1] Assuming the validity of propagation theory, it is unexpected to see the well known observation of weak results with fine hairs where pulse duration far exceeds the TRT of thin follicle and consequently the chance for heat transfer is even more. Moreover, the efficacy of older lasers (of shorter pulse durations) has been confirmed in a large percentage of cases achieving permanent long term results (for now several years).[1] This may lay the possibility that hair stem cell targeting is not the essential (or exclusive) prerequisite for permanent results or that they could be affected in one way or another by shorter pulse lasers (e.g. by transfer of heat from adjacent follicular or hair shaft melanin). The last possibility is very likely in view of the findings of the present study. Another issue is the need to consider safety as astringently as assessing efficacy. It is
for granted that better results (with more ablative effect on the hair) could be achieved by manipulating some parameters in the treatment -e.g. by escalating the dose- but at the expense of compromising safety. In the same way, the concept of increasing the pulse duration could be viewed. Two factors seem to limit the exaggerated application of this technique. First, is the need to increase the energy fluence to maintain an acceptable irradiance (density) over this wide pulse duration. Second is that the same principle of heat transfer meant to affect the adjacent bulge may also adversely affect other adjacent "innocent" structures.[29] This may be the reason why superlong-pulse lasers are usually equipped with more than one cooling technique.[7] What applies to the axillary region may not necessarily apply to other areas keeping in mind the anatomic, histologic as well as pathophysiologic peculiarities of each region.[6,30] The axilla -for instance- is characterized by very large, deep hair follicles with relatively long telogen phase and a relatively short anagen phase. Practically, it is one of the best areas to respond to laser[31], so that assessment of this area may not reveal slight differences between different laser techniques and may not reflect the situation in other hair bearing areas of the body. Conclusion
Assessment of stem cell involvement -at the research level- may provide a more rapid (and theoretically more confident) confirmation of efficacy of laser (or other modalities) in dealing with unwanted hair. It may obviate the need for lengthy follow ups and avoid the confusion due to site to site variation in the hair
biodynamic. The study could also lend some credence to the propagation theory as a more reliable basis for dealing with hair by lasers. Nevertheless, many considerations have to be addressed in future studies as regards the optimal pulse duration and its relation to the not yet settled location of the stem cells. Other considerations include the significance of secondary germs and also the essential role played by the dermal papilla. Ignoring these (or similar) issues may be among the causes of unexplained failures or lack of permanent results. Moreover, in-vivo assessment of each treatment parameter in isolation over a wide setting range and in a wide number of cases (of different colors, with different hair characteristics and in different anatomic locations) seems to be mandatory to reach firm conclusions.
References
1. Lepselter, J. and Elman, M. (2004): Biological and clinical aspects in laser hair removal J. Dermatol. Treatment. (15): 72-83
2. Liew, S.H. (2002): Laser hair removal: guidelines for management. Am. J. Clin. Dermatol. (3)2: 107-15
3. Alster, T.S. (2000): Laser-assisted hair removal: recent advances in treating darker skin phototypes. Cosmetic Dermatol. (Sept): 49-51.
4. Anderson, R.R. and Parrish, J.A. (1983): Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science (220): 524-7
5. Ort, R.J. and Dierickx, C. (2002): Laser hair removal. Semin. Cutan. Med. Surg. (21): 129-44.
6. Dierickx, C.C., Campos, V.B. and Lin, D. (1999): Influence of hair growth cycle on efficacy of laser hair removal. Lasers Surg. Med. 11 (suppl): 21
7. Rogachefsky, A.S., Silapunt, S., Goldberg, D.J. (2002): Evaluation of a new super-long-pulsed 810 nm diode laser for the removal of unwanted hair: the concept of thermal damage time. Dermatol. Surg. (28): 410-14
8. Fiskerstrand, E.J., Svaasand, L.O. and Nelson, J.S. (2003): Hair removal with long pulsed diode lasers: a comparison between two systems with different pulse structures. Lasers Surg. Med. (32): 399-404
9. Altshuler, G.B., Anderson, R.R. and Manstein, D. (2001) Extended theory of selective photothermolysis. Lasers Surg. Med. (29): 416-32
10. Chan, E., Gat, U., McNiff, J.M. and Fuchs, E. (1999): A common human skin tumour is caused by activating mutations in
ß-catenin Nature Genetics (21): 410 - 413
11. Cotsarelis, G. (2006): Gene expression profiling gets to the root of human hair follicle stem cells. J Clin. Invest. (116)1: 19-22
12. Graphpad software downloaded from the website: http://www.graphpad.com/quickcalcs/ttest1.cfm
13. Graphpad software downloaded from the website: http://calculators.stat.ucla.edu/correlation.php
14. Cotsarelis, G., Sun, T.T. and Lavker, R.M. (1990): Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell (61): 1329-37
15. Watt, F.M. and Hogan, B.L.M. (2000): Out of Eden: stem cells and their niches. Science. (287): 1427-30.
16. Oliver, R.F. (1967): The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. J. Embryol. Exp. Morph. (18): 46-51
17. Holecek, B.U. and Ackerman, A.B. (1993): Bulge-activation hypothesis: is it valid? Am. J. Dermatol. (15): 235-57
18. Oliver, R.F. (1970): The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. J. Embryol. Exp. Morphol. (23): 219?236
19. Alster, T.S. (1997): laser -assisted hair removal. In: Manual of Cutaneous laser Techniques. T.S. Alster. Philadelphia: Lippincott-Raven. Pp:127-134
20. Grossman, M.C., Dierickx, C., Farinelli, W., Flotte, T. and Andersen, R.R: (1996): Damage to hair follicles by normal-mode ruby laser pulses. J. Am. Acad. Dermatol. (35): 889-894
21. Jahoda,
C. and Reynolds, A. (2000): Skin stem cells - a hairy issue. Nature Medicine
(6): 1095-97
22. Lask, G., Eckhouse, S. and Slatkin, M. (1999): The role of laser and intense light source in photoepilation: a comparative evaluation. J. Cutan. Laser. Ther. (1): 3-13
23. Ross, E.V., Ladin, Z., Kreindel, M. and Dierickx, C. (1999): Theoretical considerations in laser hair removal. Dermatol. Clin. (17): 333-55
24. Rogachefsky, A.S., Becker, K., Weiss, G. and Goldberg, D.J. (2002): Evaluation of a long-pulsed Nd:YAG laser at different
parameters: an analysis of both fluence and pulse duration. Dermatol. Surg. (28): 932-5
25. Lorenz, S., Brunnberg, S., Landthaler, M. and Hohenleutner, U., (2002): Hair removal with the long pulsed Nd:YAG laser: a prospective study with one year follow-up. Lasers Surg. Med. (30): 127-34.
26. Ito, M., Kizawa, K., Toyoda, M., and Morohashi, M. (2002):. Label-retaining cells in the bulge region are directed to cell death after plucking, followed by healing from the surviving hair germ. J. Invest. Dermatol. (119): 1310-1316
27. Lyle S, Christofidou-SolomidouYaping M Liu David E. Albelda E S and Cotsarelis, G. (1998): The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell. Sci. (111)21: 3179-3188
28. Olsen, E. A. (1999): Methods of hair removal. J. Am. Acad. Dermatol. 143-154.
29. Lask, G., Elman, M. and Slatkine, M. (1997): Laser-assisted hair removal by selective photothermolysis. Preliminary results. Dermatol. Surg. (23):737- 9
30. Lou, W. W. and Geronemus, R. G. (2002): Dermatologic laser surgery. Semin. Cutan. Med. Surg. (21):107-28
31. Alster, T.S., Bryan, H. and Williams, C.M. (2001): Long-pulsed Nd:YAG laser-assisted hair removal in pigmented skin: a clinical and histological evaluation. Arch. Dermatol. (137)7: 885-9.
الملخص العربي
دراسة كيميائية نسيجية مناعية لخلايا
بصيلة الشعرة الجذعية وخلايا مطرق الشعر إثر علاج الشعر بالليزر:
تأثيرالتغييرات المختلفة فى خصائص النبضة
د/أحمد ابراهيم رشيد د/زينب عبد الرحمن قمر*
قسم الأمراض الجلدية والتناسلية – جامعة عين شمس
* قسم الباثولوجي – جامعة عين شمس
فى هذه الدراسة تم تعريض10 من الإناث البالغين الأصحاء لجلسات علاج الشعر
بالليزر لمنطقة الإبطين و ذلك باستخدام 7 برامج مختلفة لكل حالة (برنامج ثابت
لكل ربع من منطقة الشعر بالابط مع ترك الربع الأخير بدون علاج).
وقد أخضعت الحالات لجلستي علاج بالليزر بفارق ستة أسابيع (بطول موجة 755
نانومتر أو 1064 نانومتر وبطول نبضة مقدارها 5 أو10 أو40 أو100ميللي ثانية
ومساحة نبضة مقدارها 7 أو 12 أو 15 أو18 ميلليمتر) ومن ثم تم تقييم الحالات
إكلينيكيا بعد الجلسة الثانية بستة أسابيع وبعد ستة أشهر.
كما تم فحص الجلد بعد 6 أسابيع من أخر جلسة بالفحص النسيجي المناعي وذلك لتقييم
الخلايا الجذعية لبصيلة الشعرة وكذا خلايا مطرق الشعرة عن طريق دالات الكيراتين
الخلوى-15 CK-15 و العامل LEF-1 على الترتيب.
ولقد أظهرت الدراسة فعالية ذات مغزى لكل البرامج المستخدمة سواء بالنسبة
للتأثير الاكلينيكى على المدىالقصير والطويل أو من حيث التناقص الملاحظ فى
الخلايا الجذعية وخلايا مطرق الشعرة. الا أنه فى البرامج ذات المساحة الصغيرة
للنبضة (12مم) وأيضا تلك المستخدم فيها طول نبضة مقدارها 40 مللى ثانية كانت
النتيجة كانت أقل من نظيراتها في البرامج الأخرى بصورة ذات مغزى سواء على
المستوى الاكلينيكى أو المستوى النسيجى المناعى. كما أظهرت الدراسة نوعا من
التناسب الطردى بين المعدل الاكلينيكى القريب والبعيد.
و,بالنسبة لمعدل LEF-1 فقد أظهر تناسبا عكسيا قويا مع المعدل الاكلينيكىالقريب
وبدرجة أقل بكئير مع المعدل الاكلينيكى البعيد. كما أظهر تناسبا طرديا قويا مع
معدل الكيراتين الخلوى- CK-15
أما بالنسبة للكيراتين الخلوى-15 فد أظهر تناسبا عكسيا قويا مع كل من المعدل
الاكلينيكى القريب والبعيد وتناسبا طرديا قويا مع معدل LEF-1
و يمكن أن نقترح فى ضوء هذه النتائج أهمية استهداف الخلايا الجذعية للتأثير
الفعال على البصيلة كما يمكن اعتبار قياس الخلايا الجذعية مؤشرا يعتمد عليه
للتنبؤ بنتائج علاج الشعر على المدى الطويل
© 2006 Egyptian Dermatology Online Journal | 



