|
|
Abstract
Behcet's disease (BD) is a systemic immunoinflammatory disorder the
etiopathogenesis of which has not been clearly determined. As various functions of neutrophils in peripheral blood such as chemotaxis, phagocytosis and generation of reactive oxygen species (ROS) increase in BD, ROS-mediated oxidative stress related to neutrophil activation may have an important role in the pathogenesis of BD.
The objectives: to determine the levels of serum nitric oxide (NO)), malondialdehyde (MDA), erythrocyte superoxide dismutase (SOD) and in patients with BD and to correlate them with disease activity.
Subjects and Methods: Sixteen patients fulfilling the criteria of the international study group for
Behcet's disease (BD) and ten apparently healthy individuals matched for age and sex were included in this study.
Results The serum levels of NO, MDA and SOD in all patients (32.19
▒10.52 Ámol, 5.9 ▒0.96
Ámol and 821.3▒ 484.5 U/g Hb
respectively) were significantly higher than control group(21.1▒ 5.0
Ámol, 4.6
▒0.5 Ámol and1280▒ 244.0U/g Hb respectively) ( P<0.05) The serum levels of nitric oxide, MDA and SOD in active( 35.7▒10.11,μmol , 6.2
▒0.86 Ámol and 585▒ 262.3 U/g Hb respectively), and inactive group(24.5▒1.16
Ámol ,5.1▒0.66
Ámol and 1340▒ 251.0 U/g Hb) (t 2.21, 2.6 and 4.2, P 0.044, 0.019 and 0.001 respectively) indicating significant difference. More ever there was statistical difference between serum levels of nitric oxide, MDA and SOD in active (35.7▒10.11,μmol 6.2
▒0.86 Ámol and 585 ▒262.3 Á/g Hb respectively) and control group(21.1▒ 5.0
Ámol, 4.6
▒0.5 Ámol and1280▒ 244.0Á/g Hb respectively) (t 4.12, 5.17 and 5.2 , P 0.001,<0.001 and <0.001 respectively) While comparing serum levels NO, MDA and SOD in inactive group ((24.5▒1.16
Ámol ,5.1▒0.66 Ámol and 1340 ▒251.0 U/g Hb respectively) and control group (21.1▒ 5.0
Ámol, 4.6▒ 0.5 Ámol and1280▒ 244.0U/g Hb respectively) revealed insignificant
difference (t 1.07, 1.5 and 0.045, P >0.05 for each). Levels of NO was markedly elevated in synovial fluid (40.31▒4.7
Ámol) versus (35.7▒10.11 Ámol) in serum, this elevation in synovial fluid is correlated with serum
levels (r =0.621 P<0.001),. There was inverse correlation between serum levels of NO , MDA and SOD (r -0.665, -0.871 respectively) P<0.001. While there was positive correlation between NO and MDA (r0.562)(P<0.05), Both of them had correlation with disease activity parameters.
Conclusion: These data show that elevation of MDA&NO may be responsible for the endothelial damage in BD and contribute with lowered levels of SOD in the development of disease activity in these patients. Moreover, this study suggests that systemic elevation of NO may be a reflection of synovial elevation. Therefore, the assessment of these parameters may be a useful marker for monitoring the progress and the severity of the disease activity and management of patients with BD.
Introduction
Behcet's disease (BD) is a chronic multisystemic disorder, which is characterized by a relapsing systemic inflammatory process. In certain inflammatory conditions over production of nitric oxide (NO) could damage host cells and tissues, either directly and/or following reaction with other free radicals, such as superoxide anion to form species including peroxynitrite or hydroxyl radicals. Excessive superoxide radical production and impaired antioxidant mechanism in both the neutrophils and plasma of patients with BD have been reported [1]
Reactive oxygen species (ROS) have been proposed as a possible mediator for tissue damage associated with BD [2]. Activated leucocytes form a large number of free oxygen radicals that overwhelms the protective systems and results in cell damage and lipid peroxidation in patients with BD [3],[4] There is increase in serum and synovial 3-nitrotyrosine , a specific marker of damage induced by nitric oxide (NO) and its by-products in rheumatoid arthritis and osteoarthritis patients [5]
Ocular inflammatory disease is generally bilateral and occurs in approximately 70% of cases. Although patients with predominantly anterior uveitis have a relatively good prognosis, recurrent obliterative retinal vasculitis and thrombosis occur in 30-40% of patients and is the major cause of blindness despite vigorous treatment[6].
Systemic venous occlusion also occurs in 40% of cases One of the major factors responsible for the increased frequency of thrombogenesis is thought to be endothelial dysfunctions, which is the characteristic feature of BD [7]
The aim of the present study was to measure the levels of nitric oxide and lipid peroxidation represented by malondialdehyde (MDA) and the efficient antioxidant activity represented by superoxide dismutase (SOD) in cases of
Behcet's disease and its relation to the pathogenesis and activity of the disease.
Subjects And Methods
Subjects:
The present study was performed on 16 patients (14 males, 2 females). with BD who presented to the outpatient clinics of Dermatology & Venereology, Ophthalmology, Internal medicine and Rheumatology Departments Zagazig University Hospitals. All patients fulfilled the criteria of the international study group for
Behcet's disease [8] and the diagnosis of uveitis was made according to the International Uveitis Study Group [9]. Their ages ranged from 18 to 45 years with a mean ▒ SD(28.5▒ 4.2 years) and duration of disease ranged between 2 and 15 years with a mean ▒ SD ( 8.2▒1.5 years). In addition, 10 healthy control subjects of matched age and sex from a similar ethnic background were included in this study.
None of the patients or control subjects had received any systemic medication for at least two weeks before testing.
Determination of disease activity:
Since there was no clinically acceptable scoring system or laboratory screening profile to define the severity of BD, both clinical and laboratory findings were used to classify the patients as having active or inactive disease. Erythrocyte Sedimentation Rate (ESR), neutrophil count and acute phase reactants (a1-antitrypsin and
a2-macroglobulin) were determined and comprised the laboratory findings [10]. In clinical evaluation, patients having recurrent oral ulcerations and two of the following criteria were assumed to be in an active stage; genital ulceration, eye inflammation, skin lesion and active arthritis [11]
Methods:
Complete blood count using Cell-Dyne (Model 1700). Liver and kidney functions and lipid profiles were measured using automated clinical chemistry analyzer (ADVIA 1650). ESR was determined by the classic Westergren methods.
a1-antitrypsin and
a2-macroglobulin concentrations were measured in the serum by a
BN ProSpec (DADE BEHRING Germany) [12]
Total NO measurement was performed using an enzyme linked immunosorbent assay autoanalyser (Biosystem, Italy) and total NO kit9 (Assay Designs, Inc., Ann Arbor, MI,
USA). The results are presented in Ámol/ L) [13].
Serum MDA levels were measured according to a method described elsewhere [14] The principle of the method based on the
spectrophotometer measurement of the color occurring during the reaction of thiobarbituric acid with MDA. Concentration of thiobarbituric acid reactive substances was calculated by the absorbance coefficient of the malondialdehyde-thiobarbituric acid complex and expressed as
Ámol.
Measurement of SOD activity was performed following the method of Aydin et al [15].The results are presented in U/g Hb.
Statistical analysis :
Results were expressed as mean ▒standard deviation (SD) and were analyzed statistically by using the independent samples t-test (Student's t-test). Correlation analysis performed with Pearson correlation test. P values below 0.05 considered significant. Statistical analysis performed using SPSS-8 FOR WINDOWS computer package [16].
Results
In this study, 11 patients of 16 were in active stage whereas the remaining
five were in inactive period. Clinical findings for all patients recorded as
shown in (Table 1).
|
Item |
No of patients (16) |
Percent of cases |
|
Recurrent oral ulcer |
15 |
93.75% |
|
Recurrent genital ulcer |
11 |
68.75% |
|
Eye findings: |
8 |
50% |
|
Retinal vasculitis |
3 |
|
Uveitis |
5 |
|
Arthritis |
11 |
68.80% |
|
Oligoarthritis |
9 |
|
Monoarthritis |
2 |
|
Vascular findings: |
4 |
20% |
|
Venous: Sup.Thrombosis, DVT |
3 |
|
Arterial: aneurysm, thrombosis |
1 |
|
Skin involvement |
6 |
37.50% |
|
Papulopustular, pseudofolliculitis |
1 |
|
Erythema nodosum |
3 |
|
palpable purpura |
2 |
|
Skin Pathergy test |
4 |
25% |
Table (1) : Clinical findings of patients with
Behcet's
disease
The levels of acute phase reactants (
a1-antitrypsin
and a2-macroglobulin), ESR and neutrophil counts were significantly higher in patients with active disease than in those with inactive disease( for each P<0.01) or in control subjects (for each P<0.001) (Table 2).
|
|
Active BD |
Inactive BD |
control |
|
n=11 |
n=5 |
n=15 |
|
Neutrophil (103/ml) |
6.4 ▒
1.4
ć ç |
3.9 ▒
0.8ç |
2.6 ▒
0.3 |
|
ESR (mm/h) |
35.3 ▒
10.2
ć ç |
18.9 ▒
5.9ç |
7.48 ▒
1.85 |
|
a1-antitrypsin(mg/dl) |
232 ▒ 42
ć ç |
157 ▒
23.2ç |
120 ▒
18.1 |
|
a2-macroglobulin(mg/dl) |
279 ▒ 45
ć ç |
214 ▒ 31ç |
165 ▒ 23 |
ćsignificant different from the inactive patients (P<0.01).
ç significant different from the
control group (P<0.001).
Table
(2): Neutrophil count, erythrocyte sedimentation rate (ESR)
and acute-phase reactants (a1-antitrypsin,
a2-macroglobulin)
in patients with active, inactive Behcet's disease (BD) and
control group.
The serum levels of NO, MDA and SOD in all patients (32.19 10.52
Ámol, 5.9▒0.96 Ámol and 821.3▒484.5 U/g Hb
respectively) were significantly higher than control group(21.1▒5.0
Ámol, 4.6▒0.5 Ámol and1280▒244.0U/g Hb respectively) ( P<0.05) (Table 3).
|
|
All BD |
Control |
|
n=16 |
n=10 |
|
NO (Ámol/L) |
32.19▒10.52* |
21.13▒4.9 |
|
MDA (Ámol/L) |
5.9
▒0.96* |
4.6▒0.50 |
|
SOD
(U/g
Hb) |
821.3▒
484.5* |
1280▒244.0 |
*significant different from the control (P<0.05).
Table (3) : Statistical analysis of NO, MDA and SOD levels
between all Behcet's disease patients and control
The serum levels of nitric oxide, MDA and SOD in active(
35.7▒10.11,μmol , 6.2▒0.86
Ámol and 585▒262.3 U/g Hb
respectively), and inactive group(24.5▒1.16 Ámol ,5.1▒0.66
Ámol and 1340▒251.0 U/g Hb) (t 2.21, 2.6 and 4.2, P 0.044,
0.019 and 0.001 respectively) indicating significant
difference. More ever there was statistical difference
between serum levels of nitric oxide, MDA and SOD in active
(35.7▒10.11,μmol 6.2▒0.86
Ámol and 585▒262.3 Á/g Hb
respectively) and control group(21.1▒5.0 Ámol, 4.6▒0.5 Ámol
and1280▒ 244.0Á/g Hb respectively) (t 4.12, 5.17 and 5.2 , P
0.001,<0.001 and <0.001 respectively) (Table 4).
|
|
Active BD
n=11 |
Inactive BD
n=5 |
Control
n=10 |
|
NO (μmol/L) |
35.7▒10.11*^
|
24.5▒7.16 |
21.13▒4.94. |
|
MDA (μmol/L) |
6.2▒0.86*^
|
5.1▒0.66 |
4.6▒0.50
|
|
SOD
(U/g Hb) |
585▒262.3*^ |
1340
▒251.0
|
1280▒244.0 |
P,0.05 in comparison with control^ and * in
comparison with inactive
Table (4): Statistical
analysis of MDA, SOD and NO levels between patients with
active and inactive stage of the disease.
While comparing serum levels NO, MDA and SOD in inactive group (24.5▒1.16
Ámol ,5.1▒0.66 Ámol and 1340▒251.0 U/g Hb respectively) and control group (21.1▒5.0
Ámol, 4.6▒0.5 Ámol and1280▒244.0U/g Hb respectively) revealed insignificant difference
(t 1.07, 1.5 and 0.045, P >0.05 for each) (Table 4).
Levels of NO was markedly elevated in synovial fluid (40.31▒4.7
Ámol) versus (35.7▒10.11 Ámol) in serum, this elevation in synovial fluid is correlated with serum
levels (r =0.621 P<0.001),. There was inverse correlation between serum levels of NO , MDA and SOD (r -0.665, -0.871 respectively) P<0.001. While there was positive correlation between NO and MDA (r0.562)(P<0.05), Both of them had correlation with disease activity parameters. (Table5) ( Fig1).
|
|
r |
P |
|
MDA & NO |
0.562 |
0.003 |
|
NO & SOD |
-0.665 |
<0.001 |
|
MDA and SOD |
-0.871 |
<0.001 |
Table (5): Correlation
between MDA , SOD and NO in the active group
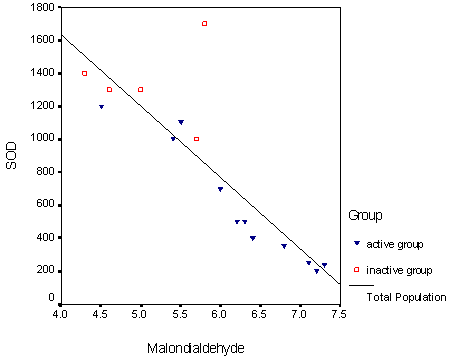
Figure(1) showed the negative correlation of SOD and
MDA in all patients.
|
|
Discussion
Vasculitis of small blood vessels is the main histopathology in BD but the factors that affect inflammation are still being investigated [17]. ROS within the human body are regarded as a major cause of inflammation and much of the microenvironmental tissue damage has been linked to the release of ROS, which have the ability to react with a range of biological molecules primarily produced by activated neutrophils at sites of inflammation [18].
Patients with BD have been shown to have increased ROS production by activated neutrophils [19], decreased superoxide scavenging activity and an impaired oxidant / antioxidant balance in plasma [20]. ROS molecules are highly reactive and capable of attacking almost every cell component, which in turn causes further damage to surrounding tissues [21].
ROS can attack double bonds in polyunsaturated fatty acids and thus induce lipid peroxidation, which in turn results in further oxidative damage. ROS mediated oxidation of cell membrane lipids leads to the formation of lipid peroxidation products such as MDA [22]
In this study neutrophil count, ESR,
a1-antitrypsin,
a2-macroglobulin levels, which are reported as markers of activity in BD, were significantly elevated in the active group as compared to the inactive group. This finding was also observed by Cekmen et al [23].
In our study we reported increased serum NO in active Behcet's patients in comparison with inactive patients and
control (P<0.05). This may be in agreement with Sancak et al. [24] and explain this increase as a part of inflammatory process that were exacerbated in active patients.
However, Orem et al [25]and Buldanlioglu et al [26] found decreased NO production in the active period of the disease as opposed to the inactive period and the control group, They thought that decreased NO production in the patients might be attributed to endothelial dysfunction in BD.
We also found increased synovial NO levels in active BD and this increase is correlated with increase of serum NO in the same patients, indicating the most probably source of increased level of serum NO from inflamed synovium this may be in agreement with Duygulu et al., [27] who found similar findings.
NO has not only an important role in initiation and perpetuation of inflammation and tissue injury but also had a role of apoptosis of chondrocytes that make Saliha et al [28] test chondroprotective effect of hyaloronan injection by its ability to reduce synovial NO levels in osteoarthritis patients
The results of the present study showed a statistically significant difference in serum MDA levels between BD patients and control group. These findings are consistent with those of Orem et al [29] who reported that the increase in MDA levels indicate increased lipid peroxidation ,confirm the increased oxidative stress and explain the decline in SOD activity in patients with active BD. Buldanlioglu et al [26] and Sandikci et al [30] have also shown that MDA levels are elevated as a result of lipid peroxidation caused by the superoxide radical which are produced in an excessive amount in
active polymorphonuclear leucocytes and plasma during the active stage of BD. This may be in agreement with our finding of positive correlation between NO and MDA (r 0.562 , P<0.05).
In our study, we determined the SOD activities in erythrocytes, which are the cells richest in antioxidant enzymes and thought to reflect tissue activity [31] We found decreased serum SOD activity in BD patients in comparison with healthy controls [32]. This finding was also reported by Tuzun et al [33] and could be attributed to the observation that Serum SOD activity is generally considered to vary in response to consumption for prevention of oxidation. So, skin and mucosal lesions have been found to be ameliorated with the treatment of SOD preparations [34].
On the other hand Buldanlioglu et a l[26] did not find any difference between the patient and control groups and between active and inactive periods of the same patients.
Pharmacological agent used in treatment of BD include both immunosuppressive and anti-inflammatory drugs most notably the corticosteroids. While these drugs are often effective in retarding the development of the disease and prolongation of the lifespan of BD patients, their long-term use is associated with considerable number of side effects [35]
Use of antioxidants in concerts with this conventional drug therapy might offer an advantage over the drug therapy alone, since drug dosage could be probably reduced, thus minimizing the side effects associated with their use [36].
Chambers et al [37] found that vitamin C intake is associated with decreased vascular damage in BD patients and is inversely associated with the activity of the disease.
Antioxidant vitamins use as ? carotene, ? tocopherol, vitamin E and selenium as adjunctive treatment with classical treatment of BD could be an important research field for amelioration or even prevention of BD [38].
In conclusion, endothelial damage and increased PMN leukocyte activity in the active stage of the disease in BD may result in a pro-oxidation environment, which in turn results in decreased antioxidant SOD activity and increased lipid peroxidation as evidenced by increased MDA levels
Moreover, this study suggests that systemic elevation of NO may be a
reflection of synovial elevation
References
1. Suzuki KM and Suzuki N et al.
Behcet's disease. Clin exp Med 2004;4(1):10-20.
2. Niwa Y, Miyake S, Sakane T et al. Auto-oxidative damage in
Behcet's disease-endothelial cell damage following the elevated oxygen
radicals generated by stimulated neutrophils. Clin Exp Immunol 1982;49: 247-255.
3. Pronai L,
Ichikawa Y, Nakazawa H et al. . Enhanced superoxide generation and the decreased superoxide scavenging activity of peripheral blood leukocytes in
Behcet's disease-effects of colchicine. Clin Exp Rheumatol 1991;9:227-233.
4. Adam B and Calikoglu E. Serum interleukin 6, procalcitonin and c-reactive protein levels in subjects with active
Behcet's disease. 2004:18:318-320.
5. Khan F, Siddiqui A. Prevalence of anti-3- nitrotyrosine antibodies in the joint synovial of patients with rheumatoid arthritis, osteoarthritis and systemic lupus erythematosus. Clin Chim Acta 2006, Mar 2,34:40.
6. Yilmaz G, Sizmaz S, Yilmaz ED, Duman S, Aydin P.: Aqueous humor nitric oxide levels in patients with
Behcet'sŞ et disease. Retina 2002 ; 22:330-335.
7. Lie JT. Vascular involvement in
Behcet's disease: arterial and venous vessels of all sizes. J Rheumatol. 1992; 19: 341 -343.
8. International Study Group for
Behcet's Disease. : Criteria for diagnosis of Behcet's disease. Lancet 1990;335:1078-80.
9. Bloch-Michel E and Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234-235.
10. Evereklioglu C, Er H, Turkoz
y et al. Serum levels of TNF-a,
sIL-2R,IL-6,and IL-8 are increased and associated with
elevated lipid peroxidation in patients with Behcet's
disease. Med Inflamm 2002;11:87-93.
11. Bekpinar SS, Kilic N, Unlucerci Y et al. Evaluation of nitrosative and oxidative stress in
Behcet's disease .JEADV 2005;19:167-171
12. Sakane T, Suzuki N and Nagafuchi H. Etiopathology of
Behcet's disease: Immunological aspects. Yonsei Medical J 1997;38(6): 350-358.
13. Torre d, Serrario G, Speranze F et al. Concentration of nitrite in serum of patient with HIV 1 infection .Journal Chem Pathol 1996; 49:574-577.
14. Jain SK. : Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochem Biophys Acta 1998;937:205-10.
15. Aydin A, Orhan H, Sayal A et al. oxidative stress and nitric oxide related parameters in type II diabetes mellitus. effect of glycemic control. Clin Biochem .2001;34:65-70 .
16. Knapp, RG and Miller. AF: Clinical epidemiology and Biostatistics. Howall Publishing CO., Pennsylvania 1992.
17. Oztas M o, Onder M, Gurer A et al. Serum interleukin 18 and
tumor necrosis factora levels are increased in
Behcet's disease. Clinical & experimental Dermatol. 2005;30:61-63.
18. Yazici C, Kose K, Calis M et al.: Increased advanced oxidation protein products in
Behcet's disease: a new activity marker. Br J Derm (2004) 151: 105-111.
19. Taysi S, Kocer I, Memiogullari R, et al. : Serum oxidant/antioxidant status in patients with
Behcet's disease. Ann Clin Lab Sci (2002);32:377-82.
20. Gunduzk, Ozturk G and Sozmen E.Y. Erythrocyte superoxide dismutase, Catalase activities and plasma nitrite and nitrate levels in patients with
Behcet's disease and recurrent aphthous stomatitis.Clin &experimental Dermatol (2004);29:176-179.
21. Kose k Yazici C Cambay N Et al.: Lipid Peroxidation and erythrocyte antioxidant enzymes in patients with
Behcet's disease J Exp Med (2002);197:9-16.
22. Karakucuk S, Baskol G , Oner AO et al. : Serum Paraoxonase activity is decreased in the active stage of
Behcet's disease. Br. J. Ophthalmol (2004); 88:1256-1258.
23. Cekmen m, Evereklioglu C, Er H et al. : Vascular endothelial growth factor levels are increased and associated with disease activity in patients with
Behcet's syndrom. Int. J. Dermatol. (2003);42,870-875.
24. Sancak B, Onder M, Oztas M O et al. Nitric oxide levels in
Behcet's disease.JEADV.2003:17:7-9.
25. Orem A, Efe H, Deger O, et al. : Relationship between lipid peroxidation and disease activity in patients with
Behcet's disease. J Dermatol Sci (1997);16:11-16.
26. Buldanlioglu S, Turkmen S, Ayabakan HB et al. : Nitric oxide, lipid peroxidation and antioxidant defence system in patients with active or inactive
Behcet's disease (2005):153:526-530.
27. Duygulu F,
Evereklioglu C, Calis M et al. Synovial nitric oxide concentrations are increased and correlated with serum levels in patients with active
Behcet's disease: a pilot study Clin Rheumatol (2005) 24: 324-330.
28. Saliha K, Ahmet K, Kadir Y et al.Effects of different hyaluronic acid products on synovial fluid NO levels in knee osteoarthritis Clin Rheumatol (2005) 24: 497-501.
29.
Írem A, Vanizor B, ăim it G et al. Decreased nitric oxide production in patients with
Behcet's disease. Dermatology 1999; 198 : 33-36. it G et al. Decreased nitric oxide production in patients with
Behcet's disease. Dermatology 1999; 198 : 33-36.
30. Sandikci R, Turkmen S, Guvenen G et al.: Lipid Peroxidation and antioxidant defence system in patients with active or inactive
Behcet's disease.Acta Derm Venereol(stockh)
(2003):83:342-346.
31. Karan A, Karan MA, Vural P et al : Synovial fluid nitric oxide levels in patients with knee osteoarthritis. Clin Rheumatol. 2003; 22:397-399.
32. Onder M, GuĘ rer MA: The multiple faces of
Behcet's disease and its aetiological factors. J Eur Acad Dermatol Venereol. 2000;15:126-136.
33. Tuzun A, Aydyn A Turan M.: Erythrocyte antioxidant activity and trace element levels in
Behcet's disease. Biol Trace Elem Res 1998;64:169-174.
34. Niva Y.: Lipid peroxides and superoxide dismutase (SOD) induction in skin inflammatory disease and treatment with SOD preparation Dermatologica 1989;179:101-106.
35. Akar A, Arca E, Serdar MA et al. Correlation between erythrocyte antioxidant activity, lipid peroxidation and disease activity in patients with
Behcet's disease.JEADV.2003; 17:469-490.
36. Kim HA, Choi KW, Song
YW. Arthropathy in
Behcet's disease. Scand J Rheumatol 1997; 26:125-129.
37. Chambers JC, Haskard DO, Kooner JS. Vascular endothelial function and oxidative stress mechanism in patients with
Behcet's syndrome. J Am Coll Cardiol. 2001; 37: 517-520.
38. Onder m Gurer MA. : The multiple faces of
Behcet's disease and its aetiological factors .J Eur Acad Dermatol Venereol.2000; 15: 126-36.
ę 2006 Egyptian Dermatology
Online Journal
|

