Abstract:
Objective: psoriasis is a chronic dermatosis characterized by the presence of heavy neutrophilic infiltrate in both the dermis and epidermis, together with elongated tortuous blood vessels in dermal papillae. Several factors are found to be behind such a type of inflammation, including PlGF. Recognition of how this factor involved in the pathogenesis of psoriasis may be of great help in the development of a new specific therapeutic modality of this disease.
Aim of the study: We aimed in this work to evaluate the role of PlGF as one of the factors underlying the pathogenesis of psoriasis and psoriatic arthritis through induction of angiogenesis and recruitment of the neutrophilic infiltrate and its correlation with disease severity.
Patients and methods: forty psoriatic patients were included in this study; examination and determination of the disease severity using PASI score and disease duration were done. Measurement of the serum PlGF level using ELISA technique was performed and correlation of disease severity and duration with its level was also done.
Results: we found that PlGF was high in 92.5% of patients and was significantly high in those with sever and moderate disease activity in comparison to those of mild activity and control group. There was also insignificant difference in its serum level between the patients with mild disease activity and control group. While, the correlation between the disease severity and its level was a statistically significant positive correlation, the correlation between the disease duration and the serum level of PlGF was insignificant. Conclusion: PlGF has a role in the pathogenesis of psoriasis and its antagonists together with other factors that interfere with its function may be of help as a new modality in treatment of psoriasis. Introduction
Psoriasis is a genetically determined inflammatory and proliferative disease of the skin [1]. It is a common chronic, recurring skin disease that is characterized by macroscopic and microscopic skin alteration[2]. Inflammation was found to have a role in the progression of a variety of diseases such as psoriasis[3].Angiogenesis and inflammation are closely linked and increased vascularity is a prominent feature of a number of inflammatory human diseases, including psoriasis and a number of inflammatory joint disease including psoriatic arthritis [4]. several angiogenic factors are mostly involved in inflammatory vascular response .among the known angiogenic factors, vascular endothelial growth factors( VEGF) which have emerged as a central regulator of angiogenic process under both physiological and pathological conditions[5]. Placenta growth factor (PlGF) has been described as a secreted growth factor with strong homology to VEGF based on amino acids and cDNA sequences. It is a member of the (VEGF) family, comprising at least five cytokines especially involved in the regulation of vascular endothelium differentiation[6]. It plays an important role in promoting adult pathological angiogenesis through synergizing with VEGF. Such evidence opens the possibility of employing PlGF for therapeutic modulation of skin angiogenesis [7]. For this reason and because of its role in the pathogenesis, and its promising future as a therapeutic option of psoriasis, we studied the level of PlGF in the serum of psoriatic patients, correlated it with the disease severity and duration, as most of the studies were performed in vitro and on animal models.
Patients and methods: This study has been carried out on forty psoriatic patients (25 females and 15 males) attended the dermatology outpatient clinic Ain Shams University hospital .Their age ranged from 21- 54 and they were diagnosed clinically by their characteristic skin lesion. Ten healthy volunteers (6males and 4 females) matching the patients age and sex, served as a control group. Patients with recent myocardial infarction, tumor growth, wound healing and diabetics were excluded from this study (all have abnormal PlGF level). All patients were not on treatment for at least one month, they were all subjected to full history taking and thorough clinical examination including general and local skin, nail and joint examination. Severity of the lesions was evaluated using the PASI score (Psoriasis Area and Severity Index) proposed by Fredrikssen and Patterson in1978 [8] as follows:
1- The head (H) corresponding to 10% of body surface area (BSA).
2- The trunk (T) corresponding to 30% of BSA.
3- The upper extremities (U) corresponding to 20% of BSA.
4- The lower extremities (L) corresponding to 40% of BSA. According to the extent each area of psoriatic involvement (A) was given a numerical value from (0-6) corresponding to the following scale:
0=none. 1=<10%. 2=>10%-30%.
3=>30%-50%. 4=>50%-70%.
5=>70%-90%. 6=>90%-100%. In each area, the erythema (E), infiltration (I) and desquamation (D) were also assessed, subjectively on (0-4) scale as follows:
0=none. 1=mild. 2=moderate.
3=sever. 4=very sever. The PASI score was then calculated from the following formula:
PASI= [(EH+IH+DH)xAH] x 0.1
+ [(ET+IT+Dr)xAT] x 0.3
+ [(EU+IU+DU)] x AU] x 0.2
+ [(EL+IL+DL)] x AL] x 0.4 The disease severity was considered as: - Mild: if PASI score is less than 15.
- Moderate: if PASI score is from 15- 25.
- Sever: if PASI score is more than 25. Joint were examined for tenderness, hotness, swelling, subcutaneous nodules, effusion, deformity, muscle wasting, active and passive movement.
Sample collection and storage: Ten ml blood samples were collected from each patient and from each one of the control group. Samples were allowed to clot for 30 minutes at room temperature then centrifuged for 10 minutes at 5000 rpm. Serum was removed in aliquots and stored at - 20?c.
Measurement of serum PlGF using ELISA: PlGF levels were measured by ELISA using a combination of a monoclonal (MoAB) and biotinylated polyclonal antibodies specific for PlGF. These antibodies were selected as they show no cross reactivity with VEGF. Plates were coated with 100 ul of 2.5 ug/ml MoAB of PlGF in 0.05 molar (m) carbonate buffer PH 9.6, over night at 4?c. Plates were blocked for a minimum of 1 h at room temperature with 300 ul of 5% sucrose, 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS), prior to addition of samples, controls and standards. A standard curve ranging from 30 pg/ml to 4 ng/ml was produced using rPlGF diluted in ELISA buffer (0.1 BSA, 0.05% Tween 20 in TBS). Positive and negative control samples of rPlGF at 1 ng/ml were added to every plate. The plates were then incubated for 2 h prior to the addition of 100ul of 150 ng/ml biotinylated polyclonal antibody to PlGF for 2 h. Detection involved 100 ul streptavidin peroxidase diluted 1:6000 for 25 min, then 100 ul of TMB substrate for 10 min. The reaction was stopped by the addition of 50 ul 0.5 m sulphoric acid. Plates were then read at 450 nanometer. All incubations were carried out at room temperature except coating, and plates were washed six times with 400 ul of 0.1 m PBS with 0.05% Tween after each step. Samples were measured in duplicate and mean values calculated using softmax software. The intra- and interpolate coefficients of variation were 23.5% and 18%. The assay was shown to be specific for PlGF with positive control samples only recording background levels.
Statistical analysis: Both clinical and laboratory data were recorded, windows xp with excel and SPSS were used to calculate the mean and standard deviation. Coefficient of correlation between the disease severity, duration and the level of PlGF were calculated using person's method, with P value considered significant if <0.05 and highly significant if < 0.001. Results:
This study was conducted on 40 (25 females and 15 males) psoriatic patients, ten of these patients have psoriatic arthropathy (PsA), with their age ranged from 21 -54 years old (mean 36.42 ± 7.69). It was found that 30% of our patients have psoriatic arthritis (12 patients); 8 patients presented by oligoarthritis (20%), 3 patients by polyarthritis (7.5%) with distal interphalangeal joints affection and only one patient (2.5 %) presented by spondyloarthritis occurred simultaneously with the skin lesions. The patients were divided into 3 groups according to severity of their lesions assessed using PASI score and the fourth group was the control group as follows:
Group I: were 25 patients, 9 of them have PsA, with their PASI score range 28 - 84 (mean 53.2 ± 17.41) and the duration of their disease 1- 30 years (mean 11.92 ± 7.78).
Group II: were 10 patients, 3 of them have PsA, with their PASI score range 16 - 24 (mean 18.4 ± 3.09) and the duration of their lesions 0.5 - 15 years (mean 5.75 ± 3.10).
Group III: were 5 patients with their PASI score range 6 - 12 (mean 9.2 ± 3.03) and the duration of their lesions 2 m (0.33) - 14 years (mean 6.9± 5.7).
Control group: 10 healthy volunteers with their age and sex matching the previous 3 groups. Articular pattern and examination in PsA are shown in (table 1). Table (1): Articular assessment of PsA patients.
|
Clinical Pattern |
No. of Patients |
(%) |
Examination |
No. of Patients |
(%) |
|
A symp. oligoarthritis |
6 |
60 |
Tenderness |
12 |
100 |
|
Symp. polyarthritis |
2 |
20 |
Limited ROM |
6 |
50 |
|
DIP joints affection |
1 |
10 |
Effusion |
4 |
30 |
|
Spondyloarthropathy |
1 |
10 |
Deformity |
5 |
40 |
ROM: Range of movement PlGF was found to be high in 37 (92.5%) out of the 40 patients included in this study. But none of the control group showed any elevation in the level of this angiogenic factor. Comparison between group I and group II as regard laboratory data revealed that there was a highly statistically significant difference in the serum level of PlGF between the 2 groups with P<0.001 ( mean of the 1st is 0.71 ± 0.10 and the 2nd is 0.40 ± 0.05 respectively) . There was also a highly statistically significant difference in the serum level of PlGF between group II and group III (mean 0.40 ± 0.05 and 0.25 ± 0.05 respectively) with P< 0.001. At the same time, the difference in the serum level between group I and group III was statistically highly significant (mean 0.71±0.10 and 0.25± 0.05 respectively) with p< 0.001. On the other hand comparison between group III and the control group, showed no statistically significant difference in the serum level of PlGF (mean 0.25 ± 0.05 and 0.21± 0.07 respectively) with P> 0.05. The difference in the serum level of PlGF between both group II, III and the control group was also statistically highly significant with P<0.001. Fig.1 There are also a significant positive correlation between the serum level of PlGf and the severity of the lesion with r = 0.50 and p<0.05, and the correlation between the disease duration and the serum level of PlGf was also positive but insignificant with r = 0.019 with P>0.05. Fig.2
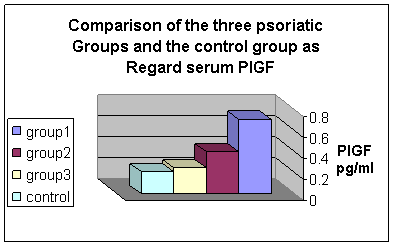
| Fig 1: Comparison
of the serum level of PIGF in the three studied groups and control group |
|
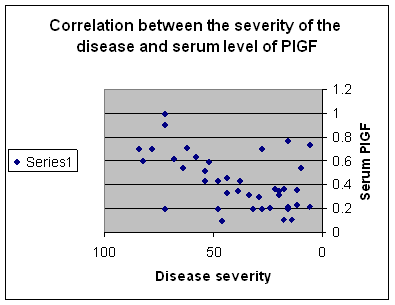
| Fig
2: Correlation between disease severities using PASI score and serum level of PIGF in all the studied groups. |
|
Discussion:
Psoriasis is a common chronic inflammatory skin disorder with a genetic susceptibility and an immunology based etiology [9]. Angiogenesis and inflammation are closely linked and accordingly increased vascularity is a prominent feature in psoriasis. The predominant type of angiogenesis observed in such an inflammation, consists of vascular enlargement of the pre-existing vessels rather than the formation of new blood vessels[10]. Psoriatic arthritis is an inflammatory joint disease that affects 10% of patients with psoriasis. Many studies showed that PsA tissues have significantly less synovial lining cell hyperplasia and greater vascularity than RA synovium[11]. Angiogenesis is implicated in the pathogenesis of psoriatic arthritis[12]. Several lines of therapy for this inflammatory skin disease have been under research for many years. Recently a new project concerning the attack of angiogenesis in this disease was brought up. We performed our study to assess the level of PlGF as an angiogenic agent that has a role in the development of such an angiogenesis, in addition to its supportive role for VEGF[7] in the pathogenesis of psoriasis. In addition, we correlated the level of PlGF with the disease duration and severity (using PASI score). In addition, angiogenesis appears to be a first-order event in psoriatic arthritis (PsA). Among angiogenic factors, the cytokines vascular endothelial growth factor, epidermal growth factor and fibroblast growth factors 1 and 2 play a central role in the initiation of angiogenesis. Most of these cytokines have been shown to be upregulated in or associated with psoriasis, rheumatoid arthritis or ankylosing spondylitis. The T allele of VEGF in +936 may act as a protective allele in the development of PsA. Further studies regarding the role of pro-angiogenic markers in PsA are warranted [13]. Placenta growth factor (PlGF) is approximately 50% identical to VEGF in the platelet- derived growth factor like domain. It occurs in 3 isoforms; 1, 2 and 3 due to alternative mRNA splicing. PlGF1 and PlGF2 but not PlGF3, were found to be expressed in human skin cells and in angiogenic human skin[4]. PIGF may be important in driving the pathology of inflammatory joints either directly by being chemoattractant to monocytes or indirectly enhancing the effect of VEGF It was found to target the basal keratinocytes and the outer root sheath follicular keratinocytes with the pattern of K14 expression [14]. It plays an important role in the induction of cutaneous inflammation and edema formation through stimulation of vascular remodeling by increasing the size and length of vessels with no major difference in vascular density [4]. This is the case of the blood vessels in psoriatic skin [15]. In this study, we found that 37 (92.5%) out of the 40 patients have increased level of PlGF. Two of these patients were included in the second group of patients and the non elevated level may be due to the increased disease activity that make PlGF binds more to VEGFR-1 leaving the serum with the ordinary level comparable to the control group. However, the third patient (who was included in the third group) the normal level of PlGF was attributed to the mild disease activity. Our results revealed that PsA was present in 30% of our patients, 20% presented by oligoarthritis, 7.5% by polyarthritis and 2.5% by spondyloarthritis. We found also that the level of PlGF showed statistically highly significant difference (p<0.001) in comparison between the 1st and both the 2nd and 3rd groups (mean 0.71 ± 0.10, 0.40 ± 0.05 and 0.25 ± 0.05 respectively). Same results were found between the 2nd and 3rd groups and between the both the 1st and the 2nd group in comparison with the control group. On the other hand, comparing the mean level of PlGF of the 3rd group with the control group showed no statistically significant difference (mean 0.25 ± 0.05 and 0.21± 0.07 respectively) with P> 0.05. We couldn't compare our results with others as no previous reference for measuring the level of PlGF in serum of psoriatic patients. However, VEGF was found to be high in serum of patients with psoriasis[16], and Carmeliet et al [17] reported that VEGF mediated angiogenesis is dependent on the presence of PlGF, accordingly, the later may be implicated in both physiological and pathological angiogenesis. This explains our findings of elevated PlGF level in serum of patients with psoriasis. Our findings that the level of PlGF is significantly high in comparison with the control group was in agreement with the results of Oura el al., [4] who reported that PlGF plays a direct role in the control of cutaneous inflammatory response after detection of it in the transgenic mice skin with inflicted inflammation. Our findings that there is a positive correlation between the disease severity and the level of PlGF with r = 0.50 and p<0.05, also supports Carmeliet et al., [17] and Oura et al., [4] opinion that PlGF has both supportive role for VEGD and direct distinct role (respectively) in the induction of angiogenesis in inflammatory conditions. However, the correlation between the disease duration and PlGF r = 0.019 was statistically insignificant with p>0.05, implying the relation of PlGF level to the activity of the disease rather than the duration of the disease. VEGF exerts its effect through binding to VEGFR1 and VEGFR2 which were found to be expressed on endothelial cells, monocytes and neutrophils. PlGF which is a member of VEGF family binds to and perform its function through VEGF1, and was found to have a mitogenic potential on endothelial cells, and stimulating effect to chemotaxis of monocytes and neutrophils [18,19]. This fact explains the elongated tortuous vessels and the neutrophilic infiltrate of psoriatic skin. It was reported also that, PlGF deficiency result in a diminished and abbreviated inflammatory response [20]. Accordingly, specific modulation of PlGF is a suggested rational therapeutic target for inhibition of angiogenesis in inflammatory diseases. This may be achieved through the use of neutralizing anti-PlGF antibodies, recombinant soluble VEGFR-1 which would potentially neutralize this growth factor and/or a signal transduction inhibitor that block PlGF receptor triggering [20,21]. Furthermore, PlGF-1 the spliced isoform of PlGF gene, was found to antagonize the VEGF induced angiogenesis through making a heterodimer with it ( PlGF-1/VEGF), that lead to depletion of the majority of VEGF homodimer, acting as a natural antagonist of VEGF when synthesized in the same population of cells [22,23]. In conclusion, PlGF may play a major role in the pathogenesis of psoriasis and PsA and its inhibition may constitutes a potential candidate for therapeutic modulation of angiogenesis and inflammation in this chronic disease .In addition and as recommended by Gottlieb 2007[24] assessment for joint disease in psoriasis patients being treated at dermatology clinic may facilitate earlier PsA diagnosis and treatment initiation, which may prevent disability and other negative impacts , However, further studies on larger scale of population and correlation of the serum level and human tissue expression of this growth factor in psoriatic patients are needed for further steps towards the proper management of this disease. References
1. Camp RD (1998): Textbook of dermatology. Edited by: Champion RH, Burton JL, Burner DA and Breathnach SM. 6th edition. Oxford, Blackwell scientific publication: 1589 - 1649.
2. Philipps, Wolk K, Kreutzer S, Wallace E et al., (2006): The evaluation of psoriasis therapy with biologics leads to a revision of the current view of the pathogenesis of this disorder. Expert Opin Ther Targets; 10(6): 817 - 831.
3. Krishnamourthy S and Honn KV (2006): Inflammation and disease progression. Cancer Metastasis Rev. 2006 Sep;25(3):481-91.
4. Oura H, Bertoncini J, Velasco P et al., (2003): A critical role of placenta growth factor in the induction of inflammation and edema formation. Blood; 101(2): 560- 567.
5. Yonekura H, Sakurai S, liu X et al.,(1999): Placental growth factor and vascular growth factor B and \c expression in microvascular endothelial cells and pericytes. J Biol Chem; 274: 35172 - 35178.
6. Odorisio T, Schietroma C, Zaccaria ML et al., (2002): Mice overexpression of placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Science; 115: 2559-2567.
7. Odorisio T, Cianfarani F, Failla CM et al., (2006): The placenta growth factor in skin angiogenesis. J Dermatol Sci; 41(1): 11-19.
8. Fredericksson T and Petterson U (1978): sever psoriasis oral therapy with a new retinoid. Dermatologica; 157: 238 - 242.
9. Michael R and Alan J (2006): Immunopathogenesis of psoriasis. Australasian J dermatol; 47: 151- 159.
10. Thurston G, Rudge JS, and Ioffe E et al., (2000): Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med; 6: 460 - 463.
11. Veale DJ, Fearon U, Fraser A, Reece R and Emery P (2000): Angiogenesis growth factors and vascularity in early psoriatic arthritis. J Rheumatol; 27 suppl. 59: 11.
12. Kuroda K, Spadin A, Shoji T, Fleischmajer R and Lebwohl M (2001): Altered expression of angiogenesis and Tie 2 endothelium receptor in psoriasis. J Invest. Dermatol; 116(5): 713 - 720.
13. Butt C, Lim S, Greenwood C and Rahman P (2007): VEGF, FGF1, FGF2 and EGF gene polymorphisms and psoriatic arthritis. BMC Musculoskelet Disord; 8: 1.
14. Vassar R, Rosenberg M, Ross S et al., (1989): Tissue specific and differentiation specific expression of a human k14 keratin gene in transgenic mice. Proc Natl Acad Sci USA; 86: 1563 - 15670
15. Braverman IM and Sibley J (1982): Role of the microcirculation in the treatment and pathogenesis of psoriasis. J Invest Dermatol; 78: 12 - 17.
16. Bhushan M, McLuaghlin B, and Weiss JB et al., (1999): levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Dermatol; 141 (6): 1054 - 1060.
17. Carmeliet P, Moons L, Luttun A et al., (2001): Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med; 7:575- 583.
18. Creamer D, Allen M, Jaggar R et al., (2002): Mediation of systemic vascular hyperpermeability in sever psoriasis by circulating vascular endothelia growth factor. Arch Dermatol; 138 (6) : 791 - 7960
19. Park JE, Chen HH, Winer J et al., (1994) : Placenta growth factor- potentiation of vascular endothelial growth factor bioactivity in vitro and in vivo and high affinity binding to Flt-1 but not to FLK-1/KDR .J Biol Chem; 269 : 25646-25654.
20. Cianfarani F, Zambruno G, and Broqelli L et al., (2006): Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential. Am J Pathol; 169 (4): 1167- 1182.
21. Bottomley MJ, Webb NJA, and Watson CJ et al., (2000): Placenta growth factor (PlGF) induces vascular endothelial growth factor (VEGF) secretion from mononuclear cells and is co-expressed with VEGF in synovial fluid. J Clin Exp Immunol; 119: 182- 188.
22. ZhengY, Murakami M, Takahashi H et al., (2006): Chimeric VEGF-E (NZ7)/PlGF promotes angiogenesis via VEGFR-2 without significant enhancement of vascular permeability and inflammation. Arterioscler Thromb Vasc Biol; 26(9): 2019 - 2026.
23. Eriksson A, Cao R, and Pawliuk R et al., (2002): Placenta growth factor-1 antagonizes the VEGF induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell; 1(1): 99 -108.
24. Gottlieb AB, Mease PJ, Mark Jackson J et al., (2007): Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists' offices. J dermatol treat; 17 (5): 279 - 287.© 2007 Egyptian Dermatology Online Journal | 

