|
|
Abstract:
Primary cutaneous leiomyosarcoma is an uncommon malignant neoplasm with a predilection for the lower extremities. A retrospective study of four cases was undertaken to analyze clinicopathological characteristics and immunohistochemical profile of these neoplasms with special emphasis on prognosis. Two male and two female patients aged between 49 and 80 years, presented with a painless
tumor involving the lower lip, the chin, the scrotum and the shoulder. Histological examination of the biopsy specimen established the diagnosis of cutaneous leiomyosarcoma. All cases coexpressed smooth muscle actin and vimentin regardless of site. Surgical excision of the
tumor was performed in only three cases, one patient refused treatment. During the postoperative follow-up period, local recurrence occurred in one case. No metastases were observed. Long-term follow-up of patients with cutaneous leiomyosarcoma is mandatory so as to detect local recurrence and distant metastasis which can occur years after the initial excision.
Introduction:
Primary cutaneous leiomyosarcoma (PCL) of the skin is a rare soft tissue
tumor that accounts for about 2-3% of all superficial soft tissue sarcomas [1]. It may occur anywhere on the body with a predilection for the lower limbs. Leiomyosarcomas of the face and the scrotum are exceedingly rare. We report 4 cases of PCL particular by their unusual locations involving the face, the scrotum and the shoulder. Our aim was to highlight histological features and immunohistochemical profile of this uncommon neoplasm with special emphasis on prognostic factors.
Patients And Methods:
Over the 7-year period from January 2000 to December 2006, four cases of primary cutaneous leiomyosarcomas were diagnosed at the pathology department of La Rabta hospital. Medical records, histopathological reports and microscopic slides were available in all cases and were retrospectively reviewed. Follow-up data regarding clinical course was obtained from the patients' records. The following histopathological features in the lesions were evaluated: architectural pattern, extension to subcutaneous fat, cellularity, mitotic activity including atypical mitoses, the presence of
tumor necrosis and giant cells. The histopathological grade was evaluated according to the National Federation of
Centers Against Cancer (Fédération Nationale des Centers de Lutte Contre le Cancer) score, taking into account the percentage of necrosis (score 1 or 2), mitotic index (score 1-3) and
tumor differentiation (score 1-3)]. Grades were obtained by adding together the scores for each variable, Grade I - 2 or 3; Grade II - 4 or 5; Grade III - 6 to 8.
Results:
Clinical Findings: The clinical findings of cutaneous leiomyosarcoma in our study are summarized in
Table 1. There were two male and two female patients aged between 49 and 80 years (mean age = 63 years). Two lesions were located in the head region (lower lip and chin), whereas the two others involved the shoulder and the scrotum. They measured from 2 to 4 cm in greatest diameter. They mainly occurred as a solitary painless nodule. Ulceration was noted in two cases (cases 2 and 3). All lesions involved primarily the skin since clinical examination and radiological investigations did not disclose a
tumor elsewhere. Wide surgical excision of the tumor was performed in 3 cases. One patient refused surgical treatment.
Table 1: Clinical data and follow-up in 4 patients with cutaneous
leiomyosarcoma
|
|
Case 1 |
Case 2 |
Case 3 |
Case 4 |
|
Age / Sex |
80 / F |
69 / M |
49 / M |
55 / F |
|
Location |
chin |
scrotum |
Lower lip |
shoulder |
|
Diameter (cm) |
2 |
3,5 |
2 |
4 |
|
Clinical presentation |
Painless nodule beneath
normal epidermis |
Painless nodule with
ulceration |
Painless nodule with
ulceration |
Painless erythematous
nodule |
| |
|
|
|
|
Treatment |
Refused treatment |
Wide local excision |
Wide local excision |
Local excision |
|
Follow-up |
Lost to follow-up |
8 months |
24 months |
12 months |
|
Evolution |
? |
No recurrence |
No recurrence |
Recurrence 12 months
after surgery |
|
No metastasis |
No metastasis |
F: female, M: male.Histological Findings: The histological findings of our 4 cases are summarized in
table 2. Histological examination of the biopsy specimen established
the diagnosis of cutaneous leiomyosarcoma in all cases. All lesions showed a
nodular growth pattern. The tumor was confined to the skin and occupied
predominantly the superficial and reticular dermis in 3 cases (cases 1, 2
&3) but extended into the subcutaneous tissue in one case (case 4). The
diagnosis of leiomyosarcoma was established based on the cellularity,
increased mitotic activity and focal areas of necrosis. The tumors were
highly cellular and showed densely packed spindle-shaped and oval cells
arranged in transverse and longitudinal intersecting fascicles (figure 1 and
2). Table 2: Histological Findings in 4 cases of cutaneous
leiomyosarcoma
|
|
Case 1 |
Case 2 |
Case 3 |
Case 4 |
|
Growth pattern |
Nodular |
Nodular |
Nodular |
Nodular |
|
Cellularity |
+++ |
+++ |
+++ |
+++ |
|
Atypia |
Moderate |
Marked |
Mild |
Moderate |
|
Giant multinucleate cells |
- |
+ |
- |
+ |
|
Mitotic index / 10 HPF |
15 |
25 |
4 |
17 |
|
Necrosis |
+ (<50%) |
- |
+ (<50%) |
- |
|
Ulceration |
- |
+ |
+ |
- |
|
Extension to subcutaneous fat |
- |
- |
- |
+ |
|
Histological Grade (FNCLCC) |
Grade 2 |
Grade 2 |
Grade 2 |
Grade 2 |
+++: high cellularity
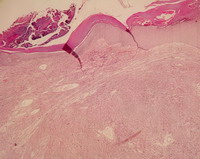
| Fig 1:
Cutaneous leiomyosarcoma with a nodular growth pattern
(Hematoxylin and Eosin, original
magnification x 10). |
|
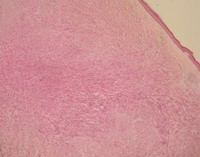 | Fig
2:
Tumour cells are arranged in transverse and longitudinal
intersecting fascicles (Hematoxylin
and Eosin, original magnification x 10). |
|
Cytomorphologically, the spindle cells showed blunt-ended nuclei and eosinophilic cytoplasm. Atypia was mild in case 1, moderate in cases 3 and 4 and marked in case 2
(figure 3). Generally, the neoplasms revealed numerous mitotic figures, some of which were abnormal. In addition, several single necrotic cells and sometimes extensive necrotic areas were also present (cases 1 and 3).
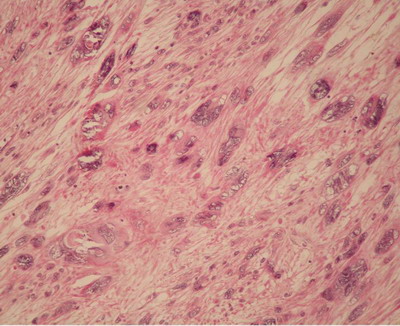 | Fig
3:
Cutaneous leiomyosarcoma with marked cytological
atypia (Hematoxylin and Eosin, original
magnification x 40). |
|
Immunohistochemical Findings: In all cases, the tumor cells were immunoreactive for smooth muscle actin
(figure 4) and vimentin but were negative for cytokeratin and CD 34. In cases 2 and 4, there was a focal positive reaction for desmin. In case 3, some
tumor cells expressed S-100 protein.
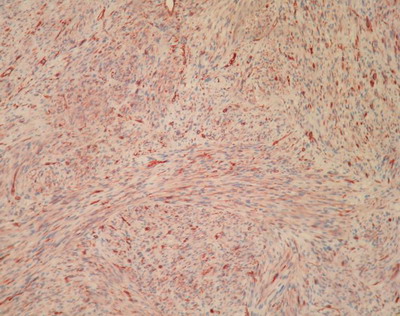 | Fig
4: Cutaneous leiomyosarcoma with
characteristic strong positivity for smooth muscle actin (SMA) (Immunohistochemistry,
original magnification x 40). |
|
Follow-up: Details of clinical follow-up were obtained in 3 patients. Only one patient was lost to follow-up (case 1). All patients were examined thoroughly for evidence of local recurrences and distant metastases. The mean follow-up period was 11 months. Cases 2 and 3 were well and alive with no evidence of recurrence or metastasis at 8 months and 24 months respectively. One patient (case 4) developed local recurrence 12 months postoperatively. No metastases have been observed during the follow-up period that ranged between 8 and 24 months. However, the follow-up period is probably too short to give a definitive evaluation
Discussion
Primary cutaneous leiomyosarcomas (PCL) are uncommon soft tissue tumors with more than 100 cases reported in the literature [2]. They account for about 2-3% of all superficial soft tissue sarcomas and are usually located on the extremities with a predilection for hair-bearing surfaces [1,2]. Fifty to 75% of lesions occur on the legs, 20-30% on the arms and only 10-15% of lesions occur on the trunk [1].
Tumors of the face (1-5%) and of the scrotum are especially rare [1]. Only six cases arising in the lips have been described in literature [3]. In cases 2 and 3, the
tumor involved respectively the scrotum and the lower lip. Atypical localizations have been described involving the penis, the breast, the orbit and the external auditory canal [4,5]. The
tumor commonly arises between the ages of 50 and 70 years with a male predominance [1,6]. Our patients were aged between 49 and 80 years (mean age = 63.25) with no sex predilection. The most common predisposing factors reported for PCL are trauma and radiotherapy [1,5,and
6]. Malignant transformation of a leiomyoma has also been reported [7]. Possible trauma was reported by one of our patients (case 4). Clinical appearance of PCL is non-specific with a wide range of differential diagnoses including squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma [5]. Dermal leiomyosarcoma is usually seen as a solitary nodule ranging from 0.4 to 6cm [5]. It often shows a pink-red to deep-red
color but may be hypopigmented, tan or bluish-black. The nodule can be lobulated, pedunculated or umbilicated. Its surface can be smooth, indurated, ulcerated, scaly, verrucous or hemorrhagic [5]. Leiomyosarcomas are divided into two subtypes depending on the location. The superficial dermal form of leiomyosarcoma is thought to arise from the arrector pili muscles whereas the deep subcutaneous type is thought to arise from the smooth muscle of the vascular wall [1,2]. Rare cases derived from areolar smooth muscle in the nipple and dartos muscle in the scrotum have been reported [2]. Lip cutaneous leiomyosarcoma may derive from ectopic sweat glands of the lips or from a hypodermic
tumor that extends to the lip [3]. Histologically, the
tumor is composed of highly cellular fascicles of spindle-shaped cells. The lesion may be relatively well-defined or poorly circumscribed. The fascicles are arranged in irregular, interlacing bundles, often intersecting at right angles. The cells have nuclei that are elongated and blunt- ended giving a "cigar" appearance. The degree of differentiation may vary within a single
tumor. In some well-differentiated areas, the cells resemble the typical smooth muscle cells of leiomyomas. Other areas may be poorly differentiated, with extensive cellular atypia and prominent nuclei and nucleoli. Mitotic figures are seen throughout the lesion. Criteria for malignancy remain controversial. Generally, accepted features of malignancy include the presence of mitoses of at least one per 10 high power fields [8], high cellularity, significant nuclear atypia and
tumor giant cells. Kaddu described two different growth patterns: a nodular pattern which is quite cellular with nuclear atypia, many mitoses and a diffuse pattern which is less cellular with well-differentiated smooth cells and inconspicuous mitoses [9]. In the presence of a small biopsy, such subtle histological features may potentially constitute a pitfall for histological diagnosis, as they may be misinterpreted as benign smooth muscle proliferation such as leiomyomas and smooth muscle hamartomas. Therefore, careful scrutiny of cytological details in multiple sections, clinicopathological correlation and immunohistochemistry are mandatory for definitive diagnosis [9]. Unusual morphologic variants of cutaneous leiomyosarcoma have been described that can introduce difficulties for diagnosis including epithelioid, granular cell, desmoplastic, inflammatory and myxoid leiomyosarcoma [2,8,10,11]. If the lesion is poorly differentiated, immunohistochemical studies can differentiate the muscular origin of the lesion. Classical immunophenotyping of PCL comprises positive vimentin, desmin and smooth muscle actin (SMA) staining. Our results confirmed positive vimentin and SMA staining in all cases. SMA seems to be more sensitive than desmin for smooth muscle
tumors, although the antibody is not always specific. Desmin staining is sensitive and specific for both normal and affected muscle tissue, but it does not differentiate between leiomyosarcoma and rhabdomyosarcoma. In our series, only two cases showed positive immunostaining with desmin. Some authors emphasize the polymorphism of the immunophenotype expression and the importance of using several antibodies [6]. Pan-muscle actin HHF35 is sometimes present focally [2]. Like desmin, this marker is not specific because it can stain myofibroblasts and striated muscle. PCL may occasionally present keratin-positive areas, or even weak EMA positivity. One case of our study was PS100-positive. Swanson suggested that there is a correlation between the site of the
tumor (dermal forms) and PS100 positivity [12]. Several benign or malignant tumoral lesions are difficult to distinguish from PCL namely desmoplastic malignant melanoma (value of PS100 and HMB45 staining), spindle-cell angiosarcoma, spindle-cell synovial sarcoma, malignant storiform pleiomorphic histiocytofibroma, schwannoma or plexiform neurofibroma and atypical fibroxanthoma. Immunohistochemistry is a valuable diagnostic tool in these difficult cases [6]. Electron microscopy can reveal intracytoplasmic myofilaments in cases which are difficult to diagnose. The most effective treatment of cutaneous leiomyosarcoma is wide excision with 3-5 cm lateral margin and a depth that includes subcutaneous tissue and fascia [13]. Local excision without adequate margins leads to recurrence and increases the risk for metastatic and possibly fatal disease. It appears to be important in all cases to ascertain that excision is complete by pathology examination because the quality of the surgical treatment influences the prognosis. An alternative method of treatment is Mohs micrographic operation to ensure complete
tumor removal [14]. Cryosurgery has also been used in elderly patients [5]. Adjuvant therapies include radiation therapy, chemotherapy and supervoltage cobalt therapy. However, leiomyosarcoma has been reported to be radioresistant; also chemotherapy with Doxorubicin was unsuccessful [1]. Recent studies have provided greater understanding of prognostic factors and the risk of recurrence. Several poor prognostic factors have been identified by Jensen namely
tumor size > = 5 cm, deep location with fascia involvement and high malignancy grade [15]. Acral distribution also appears to have a poor prognosis. While cutaneous leiomyosarcomas have been reported to show local recurrence rates of 30-50% and rarely metastasize, subcutaneous leiomyosarcomas recur in up to 70% and the metastatic rate has been reported in 30-40% of cases [16]. In summary, we report 4 cases of PCL particular by their unusual locations involving the face the scrotum and the shoulder. In the presence of a small biopsy, PCL may be misinterpreted as benign smooth muscle proliferation such as leiomyoma. Therefore, careful scrutiny of cytological details in multiple sections, clinicopathological correlation and immunohistochemistry are mandatory for definitive diagnosis. The importance of long-term follow-up needs to be emphasized. Local recurrence and distant metastasis can occur years after the initial excision. References
1. Lin JY, Tsai RY. Subcutaneous Leiomyosarcoma on the face. Dermartol Surg 1999; 25: 489-491.
2. Weedon D, Williamson RM, Patterson JW. Smooth and skeletal muscle
tumors. In: LeBoit PE, Gunter B, Weedon D, Sarasin A (eds). World Health Organization Classification of
Tumors. Pathology & Genetics. Skin Tumors. Lyon: IARC, 2006; 229-62 (251-2).
3. Baccouche D, El Euch D, Haouet S et al. Leiomyosarcoma of the lip. Ann Dermatol Venereol 2006; 133: 988-90.
4. Charlton CAC. Leiomyosarcoma of the external auditory canal. Br J Surg 1964; 51: 24-5.
5. Kuflik JH, Schwartz RA, Rothenberg J. Dermal Leiomyosarcoma. J Am Acad Dermatol 2003; 48: S51-3.
6. Auroy S, Contesso G, Spatz A et al. Léiomyosarcomes primitifs : 32 cas. Ann Dermatol Venerol 1999; 126: 235-42.
7. Tellechea O, Reis JP, Freitas JD, Masson P, Poiares A. Cutaneous leiomyosarcoma developing on a piloleiomyoma. J Dermatol 1993; 3: 28-32.
8. Choy C, Cooper A, Kossard S. Primary cutaneous diffuse leiomyosarcoma with desmoplasia. Australasian Journal of Dermatology 2006; 47, 291-295.
9. Kaddu S, Beham A, Cerroni L et al. Cutaneous leiomyosarcoma. The American Journal of Surgical Pathology 1997; 21: 979-987.
10. Suster S. Epithelioid Leiomyosarcoma of the skin and subcutaneous tissue. The American Journal of Surgical Pathology 1994; 18: 232-240.
11. Utikal J, Haus G, Poenitz N, et al. Cutaneous leiomyosarcoma with myxoid alteration arising in a setting of multiple cutaneous smooth muscle neoplasms. J Cutan Pathol 2006; 33: 20-23.
12. Swanson PE, Stanely MW, Scheithauer BW, Wick MR. Primary cutaneous leiomyosarcoma: a histological and immunohistochemical study of 9 cases with ultrastructural correlation. J Cutan Pathol 1988; 15: 129-41.
13. Fish FS. Soft tissue sarcomas in dermatology. Dermatol Surg 1996; 22: 268-73.
14. Huether MJ, Zitelli JA, Brodland DG. Mohs micrographic surgery for the treatment of spindle cell
tumors of the skin. J Am Acad Dermatol 2001; 44: 656-659.
15. Jensen ML, Jensen DM, Michalski W. Intradermal and subcutaneous leiomyosarcoma: a clinicopathological and immunohistochemical study of 41 cases. J Cutan Pathol 1996; 23: 458-63.
16. Pashaei S, Lind AC, Thomas AW, Faulkner-Jones BE. Recurrent leiomyosarcoma of the skin. Pathol Case Rev 2005; 10: 281-6.
© 2007 Egyptian Dermatology Online Journal |




