|
|
Abstract
Background Lichen planus (LP) is a chronic inflammatory disease in which liquefaction degeneration is one of the characteristic histopathological features. The relation of liquefaction degeneration and apoptosis has been reported. The aim of this study was to assess a possible role of apoptosis and its regulatory proteins in
the pathogenesis and healing of LP.
Patients and Methods Immunohistochemical methods were used to detect p53 and bcl-2 expression in skin biopsies of 10 patients with LP before and after treatment with narrow band ultraviolet B phototherapy (NB-UVB) and in 5 biopsies of normal skin as a control.
Results Strong p53 expression was detected in lesions of LP which markedly decreased after treatment with NB-UVB. On the other hand, before treatment, bcl-2 expression was less than in control specimens, but strong expression was observed in dermal lymphocytes. After treatment bcl-2 expression increased in keratinocytes and decreased in lymphocytes.
Conclusion p53 and bcl-2 may have a role in pathogenesis of LP. Apoptosis of lymphocytes may be an important mechanism of the therapeutic action of NB-UVB. Introduction
Lichen planus (LP) is a chronic inflammatory disease that appears to be related to cell-mediated immunity process [1]. Histologically, LP is characterized by hyperkeratosis, liquefaction degeneration of basal cell layer, the appearance of Civatte bodies and dense lymphocytic infiltrate in a band pattern in lamina propria [2]. The relation between the two representative histological features of LP (i.e. the liquefaction degeneration and Civatte bodies) and apoptosis has been reported and it was suggested that the Civatte bodies represent non-phagocytosed apoptotic cell fragments [3]. Apoptosis is a controlled form of cell death through the interaction of many proteins including p53 and bcl-2. bcl-2 gene is an antiapoptotic membrane associated molecule that resides in the nuclear envelope and mitochondria . p53 gene is a tumor suppressor gene that maintains genomic stability either by inducing cell cycle arrest or by apoptosis. bcl-2 is inversely related to p53 and its expression prevents apoptotic cell death [4]. Recently, narrow band ultraviolet B (NB-UVB) has been used successfully for treatment of generalized cutaneous LP [5,6,7]. The mechanism of action of NB-UVB remains hypothetical. However, the mechanism of immunomodulation by phototherapy may provide some clue and the improvement of LP by NB-UVB could be related to
photo-induced apoptosis of lymphocytes [8]. Although many studies were done on apoptosis and its regulatory proteins in oral LP [2,9-19], few studies were done on cutaneous LP [4,20,21,22]. To the best of our knowledge, no studies were done on the effect of treatment of generalized LP with NB-UVB on apoptosis and its regulatory proteins. In the present study, we investigated the expression of apoptosis regulating proteins: p53 (a positive regulator) and bcl-2 (a negative regulator) in lesions of LP before and after treatment with NB-UVB and comparing them with control skin. The aim of this study was to assess the possible role of apoptosis and its regulatory proteins in pathogenesis and healing of LP. Patients and methods
This study was conducted on 10 patients with generalized cutaneous LP at
the Outpatient Clinics of Dermatology, Zagazig University hospitals. Lesions of LP were diagnosed both clinically and histologically. Neither topical nor systemic medications of LP were taken by patients for at least 1 month before the study. All patients were treated with NB-UVB thrice weekly for a total of 40 sessions or up to clinical cure. The starting dose was 0.411 J/cm2 for patients with skin type III and 0.579 J/cm2 for patients with skin type IV. On each subsequent session, irradiation was increased by 15% unless marked erythema developed. All patients had initial skin biopsies and at the end of treatment. Five control specimens were taken from healthy subjects. All biopsies were fixed in paraffin and sectioned into 5 µm thick sections. These sections were subjected to histopathological (H&E) and immunohistochemical examination. Immunohistochemical examination
To evaluate the p53 and bcl-2 expression in the keratinocytes and lymphocytes, the sections were stained by monoclonal mouse antihuman p53 protein (N1581; DAKO Corporation,Carpinteria, CA, USA) and monoclonal mouse antihuman bcl-2 onchoprotein (N1587; DAKO Corporation) using detection system (K0673, LSAB2 system; DAKO Corporation) according to the manufacturer's instruction. The results of immune-staininig were graded according to staining intensity as absent or negative staining, weak (+), moderate (++), and strong(+++) staining. Localization of immunohistochemical staining was grouped and classified as: ''only basal layer'', ''lower 1/2 of epidermis'' and ''whole epidermis''. Results
The study included 10 patients with generalized LP (7 females and 3 males) and 5 healthy individuals as a control. The ages of patients ranged from 26 to 67 years (mean 45.40±13.10). The duration of LP ranged from 1 year to 6 years (mean 3.54±1.68). The number of NB-UVB sessions ranged from 15 to 22 sessions (mean 18.20±5.15).
p53 expression -Control skin showed weak p53 immuno-reactivity as brownish nuclear staining in basal keratinocytes in 2 specimens and negative staining in 3 specimens
(fig 1). In LP lesions, strong p53 expression was observed in basal and suprabasal keratinocytes in 8 specimens and moderate expression in 2 specimens (fig 2). -After treatment: healing lesions showed no p53 expression in basal keratinocytes in 5 specimens and weak expression in the other 5 specimens
(fig 3).
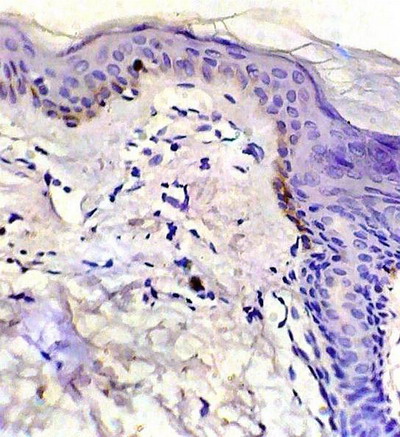 | Fig 1:
Control skin showing weak p53 expression. (ABC, Meyer s HX counter
stain x 400) |
|
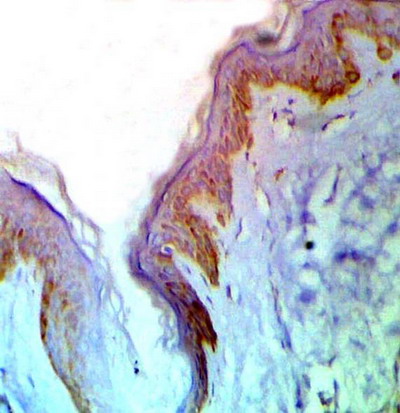 | Fig
2: A case of LP showing strong expression of p53 in basal and
suprabasal cell layers before treatment.(ABC, Meyer s HX counter
stain x 200) |
|
 | Fig
3: Weak p53 expression after treatment with NB-UVB.(ABC, Meyer
s HX counter stain x 400) |
|
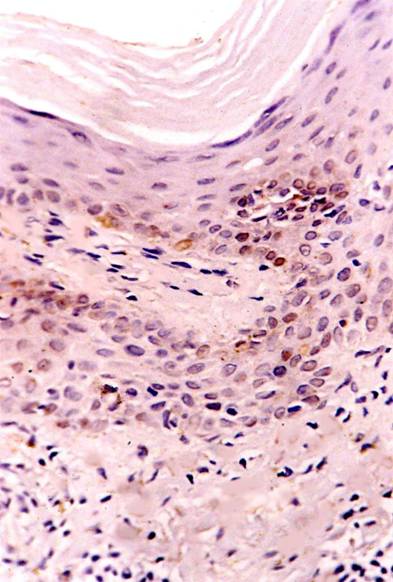
bcl-2 expression -Control skin showed weak bcl-2 immuno-reactivity as brownish cytoplasmic staining in basal layer in 2 specimens and moderate in 3 specimens
(fig 4). Dermal lymphocytes were negative for bcl-2 expression in all specimens. In LP lesions, bcl-2 was weakly expressed in the basal keratinocytes as cytoplasmic and membranous brownish staining in 4 specimens and was absent in 6 specimens. On the other hand, dermal lymphocytes showed strong bcl-2 expression in all cases (fig 5). -After treatment: increased bcl-2 expression in basal and suprabasal layers was observed in all specimens while lymphocytes showed marked decrease in bcl-2 positivity in 6 samples and negative staining in 4 samples
(fig 6).
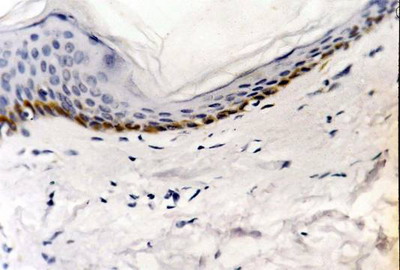 | Fig
4: Control skin showing moderate bcl-2 expression. (ABC, Meyer
s HX counter stain x 400) |
|
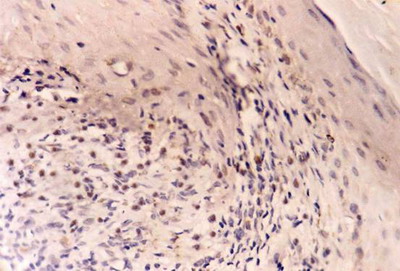 | Fig
5:
A case
of LP before treatment showing weak bcl-2 expression in basal
keratinocytes and strong expression in dermal lymphocytes. (ABC,
Meyer s HX counter stain x 400) |
|
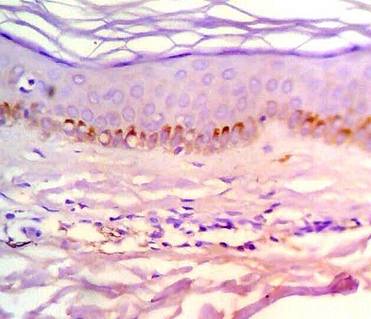 | Fig
6:
After
treatment with NB-UVB, increased bcl-2 expression in basal
keratinocytes and marked decrease in bcl-2 positivity in dermal
lymphocytes. (ABC, Meyer s HX counter stain x 400) |
|
Discussion
Liquefaction degeneration is a typical feature of the epithelium in LP [11]. Some authors consider that apoptotic phenomenon is translated into a series of cell-morphology changes designated as liquefaction degeneration produced by lymphocytes of the dermal infiltrate [10,11,12]. However, other authors consider apoptosis to be of little importance in LP and consider liquefaction degeneration an indicator of considerable cell damage that can result in cell cycle arrest, in cell senescence or more rarely, in apoptotic cell death [14,15,19]. To asses the possible role of apoptosis and its regulatory proteins in pathogenesis and healing of LP, we measured bcl-2 and p53 expression in LP lesions before and after treatment with NB-UVB and compared them with control skin. The strong expression of p53 in lesions of LP supports the notion that apoptosis is a potential mechanism of keratinocyte loss in LP [2]. p53 is induced in injured cells and maintain genomic integrity with multiple downstream targets which activate pathways of cell cycle arrest, cell repair and apoptotic cell death.[17]. Similar results were reported in oral LP [2,16,17]. Accumulation of p53 in LP associated with increased proliferation marker Ki-67 was reported by Soini et al [21]. They attributed the accumulation of p53 to be a possible response to increased proliferation rate of keratinocytes in LP or alternatively, it may be associated with apoptosis. Conversely, Hussein et al [4] reported low p53 expression in LP. They stated that they consider the low p53 expression to both protect against cellular transformation and contribute to the development of the epidermal changes in lesions of LP. However, they did not explain how the low p53 expression would achieve these effects. After treatment with NB-UVB, the healing lesions of LP showed negative and weak staining of basal keratinocytes for p53 which could be attributed to decreased epidermal proliferation and restoration of the physiologic behavior of normal skin which does not usually express p53. The low level of bcl-2 expression in LP lesions before treatment is in agreement with other authors [4,20]. Similar results were reported in oral LP [2,16,18]. The low expression of this anti-apoptotic protein might promote apoptosis of keratinocytes in LP. Hussein et al reported [4] that the low bcl-2 expression in the face of apoptosis of basal cells suggests the concomitant loss of other pro-survival molecules or increase in the pro-apoptotic molecules in the lesions of LP. Boyd et al [20] suggested that the low expression of bcl-2 in LP might indicate that bcl-2 is not prominently involved in epidermal changes in LP and the role of other members of this oncogene family needs to be elucidated. The increased expression of bcl-2 after NB-UVB treatment may be attributed to restoration of normal basal activity in the healing lesions. It is well known that in normal skin the actively proliferating cells of basal keratinocytes typically express bcl-2 which protects them against apoptotic stimuli [24]. On the other hand, the strong expression of bcl-2 in dermal lymphocytes in lesions of LP before treatment inhibits the apoptosis in lymphocytes that strengthens cell-mediated immune process causing chronicity of the disease [22]. After treatment with NB-UVB, the marked decrease in bcl-2 expression leads lymphocytes to undergo apoptosis which might contribute to healing of the lesions [25]. In conclusion, the strong expression of p53 together with the low expression of bcl-2 in lesions of LP supports the notion that apoptosis could be a potential mechanism of keratinocyte loss and that liquefaction degeneration might be a marker of apoptosis and may suggest a contributory role for these apoptosis-associated proteins in the pathogenesis of LP. The decreased bcl-2 expression in lymphocytes after treatment may provide evidence that apoptosis of lymphocytes is an important mechanism of the therapeutic action of NB-UVB in LP. Further studies on other apoptosis-associated proteins and other therapeutic modalities in different clinical types of LP are recommended. References
1.
Scully C, el-Kom M. Lichen planus: review and update on pathogenesis. J Oral
Pathol. 1985 Jul; 14(6): 431.
PMID: 3926971
2.
Dekker NP, Lozada-Nur F, Lagenaur LA, MacPhail LA, Bloom CY, Regezi JA.
Apoptosis-associated markers in oral lichen planus. J Oral Pathol Med. 1997
Apr 26(4): 170.
PMID: 9176791
3.
Kanerva L. Electron microscopic observations of dyskeratosis, apoptosis,
colloid bodies and fibrillar degeneration after skin irritation with
dithranol. J Cutan Pathol. 1990 Feb; 17(1): 37- 44.
PMID: 2319038
4.
Hussein MR, Al-Badaiwy ZH, Guirguis MN. Analysis of p53 and bcl-2 protein
expression in the non-tumorigenic, pretumorigenic, and tumorigenic
keratinocytic hyperproliferative lesions. J Cut Pathol. 2004 Nov; 31(10):
643.
PMID: 15491323
5.
Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J
Dermatol. 2002 May; 41(5): 282- 3.
PMID: 12100704
6.
Saricaolu H, Karadogan SK, Bakan EB, Tunali S. Narrowband UVB therapy in the
treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003 Oct;
19(5): 265.
PMID: 14535898
7.
Habib F, Stoebner PE, Picot E, Peyron JL, Meynadier J, Meunier L. Narrow
band UVB phototherapy in the treatment of widespread lichen planus. Ann
Dermatol Venereol. 2005 Jan; 132(1): 17. (Abstract).
PMID: 15746601
8.
Taylor A, Verhagen J, Blaser k, et al. Mechanism of immune suppression by
interleukin-10 and transforming growth factor-beta: the role of T regulatory
cells. Immunol 2006; 117: 433- 42.
PMID: 16556256
9.
Majorana A, Facchetti F, Pellegrini W, Sapelli P. Apoptosis-associated
markers in oral lichen planus. J Oral Pathol Med 1999; 28: 47- 8.
PMID: 9890458
10.
Bloor BK, Malik FK, Odell EW, Morgan PR. Quantitative assessment of
apoptosis in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod 1999; 88: 187- 95.
PMID: 10468464
11.
Tobon-Arroyave SI, Villegas-Acosta FA, Ruiz-Restrepo SM et al. Expression of
caspase-3 and structural changes associated with apoptotic cell death of
keratinocytes in oral lichen planus. Oral Dis 2004; 10: 173.
PMID: 15089928
12.
Villarroel-Dorrego M, Correnti M, Delgado R, Tapia FJ. Oral lichen planus:
immunohistology of mucosal lesions. J Oral Pathol Med 2002; 31: 410.
PMID: 12165059
13.
Bascones-Ilundain C, Gonzalez-Moles MA, Esparza G, Gil-Montoya JA, Bascones-Martinez
A. Significance of liquefaction degeneration in oral lichen planus: a study
of its relationship with apoptosis and cell cycle arrest markers. Clin Exp
Dermatol. 2007 Sep; 32(5): 556.
PMID: 17608758
14.
Gonzalez-Moles MA, Bascones-Ilundain C, Gil-Montoya JA et al. Cell cycle
regulating mechanisms in oral lichen planus: molecular bases in epithelium
predisposed to malignant transformation. Arch Oral Biol. 2006; 51: 1093.
PMID: 16914114
15.
Bascones-Ilundain C, Gonzalez-Moles MA, Esparza-Gmez G et al. Importance of
apoptotic mechanisms in inflammatory infiltrate of oral lichen planus
lesions. Anticancer Res 2006; 26: 357.
PMID: 16475718
16.
Tanda N, Mori S, Saito K et al. Expression of apoptotic signaling proteins
in leukoplakia and oral lichen planus: quantitative and topographical
studies. J Oral Pathol Med 2000; 29: 385.
PMID: 10972347
17.
Hirota M, Ito T, Okudela K et al. Cell proliferation activity and the
expression of cell cycle regulatory proteins in oral lichen planus. J Oral
Pathol Med 2002; 31: 204.
PMID: 12076323
18.
FanY, Zhan Z, Peng T, Song XL, Feng ZQ. The expression of
apoptosis-associated proteins Bcl-2, Bax in oral leukoplakia and lichen
planus. Shanghai Kou Qiang Yi Xue. 2004 Dec; 13(6): 497. (Abstract)
PMID: 15619691
19.
Bascones C, Gonzolez-Moles MA, Esparza G, et al. Apoptosis and cell cycle
arrest in oral lichen planus. Hypothesis on their possible influence on its
malignant transformation. Arch Oral Biol. 2005; 50: 873.
PMID: 16137496
20.
Boyd A, Nanney L, Cameron G, King L. Expression of bcl-2 in lichen planus,
acute graft-versus-host disease, and erythema multiforme. Am J Dermatopathol.
1997 Feb; 19(1): 46.
PMID: 9056654
21.
Soini Y, Kamel D, Paakko P, Lehto VP. Aberrant accumulation of p53
associates with ki67 and mitotic count in benign skin lesions. Br J
Dermatrol. 1994; 131: 514.
PMID: 7947202
22.
Bal N, Tuncer I, Baba M, Bolat F. Bcl-2 expression in dermal lymphocytes in
lichen planus and psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2008
May; 22(5): 640.
PMID: 18410632
23.
Laporte M, Galand P, Fokan D, de Graef C, Heenen M. Apoptosis in established
and healing psoriasis. Dermatology. 2000; 200(4): 314.
PMID: 10894962
24.
Raskin CA. Apoptosis and cutaneous biology. J Am Acad Dermatol. 1997 Jun;
36: 885-97.
PMID: 9204050
25.
El-Domyati M, Barakat M, Abdel-Razek R, El-Din Anbar T. Apoptosis, P53 and
Bcl-2 expression in response to topical calcipotriol therapy for psoriasis.
Int J Dermatol. 2007 May; 46(5): 468.
PMID: 17472673
الملخص
العربي
اعتصار(بي-53) و(بى سى ال-2) في مرض الحزاز المنسطح
بعد العلاج الضوئى بالأشعة فوق البنفسجية(ب) ضيقة النطاق
يُعتبر مرض الحزاز
المنسطح أحد الأمراض الجلدية المزمنة و الذى يظهر فى تغيراته النسجية تفسخ
وسيولة فى الخلايا التقرنية القاعدية والذى قد يوحى بحدوث هبوط انفصالى فى
الخلايا التقرنية القاعدية. ولذلك تم فى هذا البحث دراسة اعتصار البروتينات
الصاحبة والمنظمة للهبوط الانفصالى(بى-53)ل و(بى سى ال-2) فى عينات الجلد لعشرة
من المرض بداء الحزاز المنسطح قبل وبعد العلاج الضوئى بالأشعة فوق البنفسجية(ب)
ضيقة النطاق(311 نانومتر). قبل العلاج: وجد اعتصار(بى-53) عاليا فى خلايا
البشرة بينما وجد اعتصار(بى سى ال-2) اقل منه فى الحالات السوية فى خلايا
البشرة ولكنه اعلى فى الكرات الليمفاوية فى الأدمة.
بعد العلاج: قل اعتصار(بى-53) بشكل ملحوظ بينما زاد اعتصار(بى سى ال-2) فى
خلايا البشرة الى معدله الطبيعى وقل بشكل ملحوظ فى الكريات الليمفاوية فى
الأدمة.
يعكس تراكم(بى-53) ونقص(بى سى ال-2) تنشيط عملية الهبوط الانفصالى فى الخلايا
القاعدية التقرنية فى مرض الحزاز المنسطح كما يدل نقص(بى سى ال-2) فى الكرات
الليمفاوية بعد العلاج الى وجود دور للأشعة فوق البنفسجية ضيقة النطاق فى تثبيط
الجهاز المناعى وانعكاس ذلك فى تحسين المرض. وتنصح هذه الدراسة بمزيد من البحث
فى عوامل التنشيط و التثبيط الأخرى للهبوط الانفصالى فى سائر انواع الحزاز
الأخرى وتأثير طرق العلاج المختلفة عليها.
© 2008 Egyptian Dermatology Online Journal |






