|
|
Abstract
Background: As the pathogenesis of vitiligo is still obscure, its treatment is a great challenge and is often unsatisfactory. Topical calcineurin inhibitors are claimed to be a suitable option with good safety profile. Aim of the work: to evaluate the therapeutic effect of pimecrolimus on vitiligo. Patients and methods: twenty six patients with focal vitiligo are included (18 females and 8 males). The depigmented lesions extended from 5% to 20% of body surface area. Pimecrolimus cream1% was topically administered to the lesions twice daily for 3 months. Soluble IL2 receptor was measured before the start of the study and at the end. Results: of the 26 patients none had excellent response, 6 had good response, 9 had moderate response, 8 had poor response and 3 showed no response. The level of soluble IL2 receptor and the duration of the disease were inversely significantly correlated to the clinical response. No significant relation was detected between clinical response and the age of patients, family history, stress, or skin type. Better response was noticed on the limbs and trunk while the peripheral sites showed poor or no response but with no significant difference. Side effects were minimal and tolerable.
Conclusion: pimecrolimus through its immunomodulatory effect was shown to be effective in selected cases of vitiligo where phototherapy is ineffective or potentially harmful or when undesirable effects of corticosteroids are to be avoided.
Introduction
Vitiligo is an acquired pigmentary disorder characterized by depigmentation of skin and hair causing important cosmetic and psychosocial problems[1]. Its onset in at least half of the cases occurs before the age of 20[2]. There is a familial incidence of 30 %[3] and approximately 0.5 to 1%[1,4] of the world population is affected by the disease. The underlying mechanism is still unclear. Several hypotheses have been put forward including genetic predisposition[5] , neural involvement and self destruction of melanocytes (autotoxicity theory)[6,7]. There is however a growing evidence for an autoimmune mechanism involving humoral as well as cellular immunity[8,9]. Vitiligo is far more common in patients suffering from autoimmune diseases such as thyroiditis, pernicious anemia and diabetes[10,11]. Patients with vitiligo produce special melanocytes antibodies. The direct relation of these antibodies titles and the extent of vitiligo favors a direct pathogenic role of these antibodies to the disease[11]. Studies have addressed the role of peripheral blood and lesional cytokine expression in patients with vitiligo[12].
Besides, other factors such as oxidative stress, nitric oxide and
monoaminergic system are claimed to be involved in the pathogenesis. A
composite hypothesis has been suggested[7]. As the pathogenesis is still obscure, the treatment of vitiligo has generally been stressful, unsatisfactory and often disappointing and spontaneous regression of this disease is unusual[13]. It remains a challenge for the dermatologist although numerous modalities have been proposed and are currently available[14]. Conventional therapies include topical steroids, phototherapy and photochemotherapy (NBUVB and PUVA
respectively)[12,15,16]. Helium neon laser treatment may be a an option for patients with segmental vitiligo[17]. Eyebrows, eyelids and genital vitiligo are a therapeutic dilemma especially in children[3]. The adverse effects of steroids and /or unsatisfactory efficacy and difficulties for choosing any other adequate treatment are a major concern[2]. Topical calcineurin inhibitors; tacrolimus and pimecrolimus are novel topical immuno-modulatory drugs that are originally used for the treatment of atopic dermatitis[3,18,19] . When compared with conventional topical corticosteroid therapy; they have more selective mode of action, with the less adverse events related to the long term use of corticosteroids and are not associated with significant systemic absorption[13,20,21]. Preferred sites for the use of topical calcineurin inhibitors are areas such as the face, neck, flexures, and genital areas, which are more susceptible to topical corticosteroid[3,21]. The efficacy of topical calcineurin inhibitors has been demonstrated for vitiligo[22,23,24]. Calcineurin inhibitors act on T cells and mast cells inhibiting T cell activation and release of inflammatory mediators and preventing degranulation of mast cells[12,24].
Aim of the Work
The aim of this study is to investigate the therapeutic effect of pimecrolimus as an immuno-modulatory topical treatment for vitiligo.
Patients and Methods
Patients: The study involved randomly selected 26 patients with focal vitiligo
affecting no more than 20 % of body surface area depending on the hand palm rule (1%). Cases extending
for more than 20% were redirected to other generalized lines of treatment (systemic steroids, PUVA, NBUVB etc.)
Criteria for exclusion included: Cases with dermatomal vitiligo, pregnancy, lactation, women with childbearing potential not using adequate contraception method, Immunosuppresion or concomitant use of immunosuppressive medications, patients who had used any other topical or systemic treatment for vitiligo within the past 2 months, and known hypersensitivity to the used treatment .
Methods: All patients were subjected to: I- Complete history taking
including personal history, present history, family history and history with special relevance to the vitiligo condition. History of related skin or systemic disease as alopecia areata, diabetes mellitus or thyroid disease, and response to previous therapy.
II- Full clinical examination including: - General physical examination. Complete dermatological examination including site, shape, number, and distribution of lesions and the extent of involvement using the ''hand palm rule'' (size of physician's hand palm equals 1% of the total body area)[25]. The boundaries of vitiligo patches were defined using woods light. - An informed written consent was obtained before treatment. Soluble IL2 receptor (sIL2R) was measured in the serum by ELISA technique using cell free kit (T cell diagnostics, inc., Cambridge, MA,USA). It was measured before the start of the study and at the end. Pimecrolimus cream1% was topically administered to the lesions twice daily for 3 months.
VII- Clinical Assessment: - Clinical assessment consisted of clinical response determination monthly -
Monitoring for repigmentation; perifollicular pigmentation was assessed as an initial response to therapy. - The clinical response to therapy was visually scored as the percentage of repigmentation of the depigmented lesions(degree of peppering) [13] and rated as follows :
o Excellent response: If >75% repigmentation of the depigmented lesions at end of therapy. o Good response: If between 51-75% repigmentation of the depigmented lesions at end of therapy. o Moderate response: If between 26-50% repigmentation of the depigmented lesions at end of therapy. o Poor response: If <25% repigmentation of the depigmented lesions at end of therapy. o No response: If 0% repigmentation of the depigmented lesions at end of therapy.
- Evaluation of side effects each visit. - Follow-up was done for 3 months after completion of treatment to assess the stability of lesional repigmentation.
Statistics The data were analyzed using statistical package for social science SPSS version 8 software. The quantitative data are presented as mean and standard deviation. The qualitative data are presented as number and percentage. The significance of differences between study variables were evaluated using one-way analysis of variance F test and LSD, chi square, (paired t) when appropriate. P value < 0.05 was considered significant[26].
Results
I-Clinical Results: This study was conducted on 26 patients with focal vitiligo. They included 18 females and 8 males. The age ranged from 15 to 49 years with a mean 29.6 ± 7.8. The duration of disease ranged from 2.5 to 36 months with mean 21.3±9.7. Their skin types were: 8 patients (30.8 %) of skin type III, 13 patients (50 %) of skin type IV and 5 patients (19.2 %) of skin type V. In five patients (19.2 %) the disease was preceded by or correlated with psychic trauma. Four patients(15.4 %) reported positive family history. The depigmented lesions extended from 5% to 20% of body surface area. A significant clinical response to treatment was noticed. Evaluation of the clinical response showed that no patients (0 %) had excellent response, 6 patients (23.1 %) had good response
(figure 1), 9 patients (34.6 %) had moderate response (figure 2) , 8 patients (30.8 %) had poor response and 3 patients( 11.5 %) showed no response
|
|
|
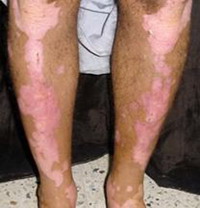
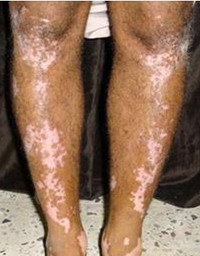
Fig 1:
A case of vitiligo on lower limbs showing good response |
|
|
|
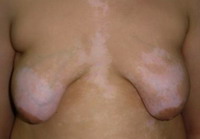
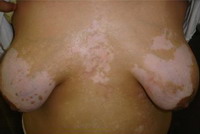
Fig
2: A case of vitiligo on the chest with moderate response |
No significant correlation was found between the clinical response and the variables, skin type, age of patients, family history or history of stress. Although we noticed that good results were more on the limbs and trunk than the peripheral parts of the body; no significant relation was present
(table 1). A significant negative correlation was detected between duration of disease and clinical response. Short duration of disease was associated with better results
(table3). Follow up evaluation for 3 months after cessation of treatment showed no recurrence of responding lesions or appearance of new lesions.
sIL2R levels: Before the start of treatment ; the level of sIL2R ranged from 550U/mL to 1500 U/mL with mean 786±142.9 After treatment the level ranged from 498 to 1428 with mean 623.0± 173.7. The level of sIL2 R significantly decreased with clinical improvement
(tables 2,3) Side effects were minimal and transient. They were reported in 5 patients in the form of mild burning sensation or pruritus. They were relieved with short courses of antihistamines and bland emollients.
|
|
No response |
poor |
moderate |
good |
P |
|
Face(no=4) |
0 0% |
1 25% |
2 50% |
1 25% |
|
|
Trunk(no=8) |
0 0% |
3 37.5% |
3 37% |
2 25% |
0.08 |
|
Limbs(no=9 |
0 0% |
3 33.3% |
3 33.3% |
3 33.3% |
(NS) |
|
Periphery(no=5) |
3 60% |
1 20% |
1 20% |
0 0% |
|
NS: non significant Table (1): The relation between the site and clinical response.
|
sIL2 R |
Before treatment |
After treatment |
P |
|
(mean±SD) for all patients |
798.1±183.8 |
623.0±173.7 |
<0.001(HS) |
HS: highly significant Table (2): The difference between sIL2R mean±SD of all patients before and after treatment.
|
P |
f |
Good response |
Moderate response |
Poor response |
No response |
|
|
|
|
|
|
|
|
Duration |
|
<0.05 |
4.23 |
16.5±12.1 |
16.5±6.5 |
29.2±4.3* |
24.3±12.0 |
mean ±SD |
|
(S) |
|
|
|
|
|
|
|
|
|
|
|
|
|
sIL2(mean±SD) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<0.01 |
6.9 |
718.3±129.6 |
751.7±82.8 |
778.1±106.3 |
1050±150** |
-before treatment |
|
(S) |
|
|
|
|
|
-after treatment |
|
<0.01 |
19.2 |
488.3±118.3 |
588.2±78.2 |
621.8±99.3 |
1001±99.9 |
|
|
(S) |
|
|
|
|
|
|
|
|
|
<0.01 |
<0.01 |
<0.01 |
>0.05 |
P***
value for paired analysis |
|
(S) |
(S) |
(S) |
(S) |
*poor response was significantly related to long duration of the disease.
**sIL2 level was statistically significantly higher among non responders .
*** No significant difference between level of sIL2 R before and after treatment in non responding cases while the three responding groups showed significant difference between levels before and after therapy.
Table (3): The relation between both duration & serum level of sIL2 Receptor and clinical response.
Discussion
As there is no gold standard treatment for vitiligo, a wide range of therapeutic options are available. When making a choice; one has to take into consideration the clinical type of vitiligo, the age of the patient, the previous experiences with the chosen therapeutic modality, the specificities of the area to be treated, and the possible side effects of the chosen therapy[3] . According to the autoimmune theory, melanocytes in vitiligo are damaged or destroyed by humoral and / or cellular immune mechanisms[8,9]. Experimental evidences suggested that the mechanism of action of calcineurin inhibitors in vitiligo may be mediated by the suppression of local cytokines and by the direct interaction of the drug with keratinocytes resulting in favourable conditions for melanocyte growth and migration[13].Calcineurin inhibitors induces proliferation of melanocytes through the interaction with
neighbouring keratinocytes[27,28]. On the other hand, there is no evidence that it also favors proliferation and /or activation of melanocytes[12]. Their main advantages compared with conventional topical corticosteroid therapy are that they are more selective in their mode of action, do not induce skin atrophy and are not associated with significant systemic absorption. Preferred sites for the use of topical calcineurin inhibitors are areas such as the face, neck, flexures, and genital areas, which are more susceptible to topical corticosteroid side effects[29]. There are various studies and considerable controversy in the results of pimecrolimus cream in vitiligo [3,30,31,32,33]. It was used in comparison with 0.05% clobetazol propionate with comparable results[23]. Pimecrolimus was proved to be of equivocal results when compared with the vehicle in generalized vitiligo[12]. On the other hand, it gave a dramatic response in a case report[30]. Good results were reported when using calcineurin inhibitors under occlusion[31]. Variable degrees of repigmentation was reported varying from excellent to moderate grades when using pimecrolimus, with more responding lesions on the trunk, elbows[32]and face[34]. To gain our experience, pemicrolimus was tried on 26 patients with focal vitiligo. Among the cases; 6 patients (23.1%) showed good and 9 patients (34.6%) showed moderate response to pemicrolimus but no excellent response was recorded. The response to treatment was not significantly related to age, family history, history of stress or skin type of the patients. However, we found that the earlier the treatment was conducted the better the response to treatment was. . The serum level of sIL2 receptor (sIL2 R) is one of the indicators of T cell function in addition to pro-inflammatory mediators as IL6 and IL8 [35]. We found that sIL2 R serum level was significantly correlated with the clinical picture and the effect of treatment. Its level significantly decreased with clinical response which indicates suppression of T cell activity and assists the immune theory of the disease. Although no significant difference in the clinical response to treatment was found between the different sites; the degree of repigmentation was relatively dependent on the site. Moderate and good response were obtained more frequently in lesions located on the limbs and trunk as compared with lesions located on the peripheral area as fingers where the response was disappointing (3 out of 5 cases of vitiligo on the periphery) didn't respond at all and the
remaining 2 were poor responders
(table 1). We found no significant effect of skin type on the response. Alternatively, in another study a faster improvement was associated with Fitzpatrick skin types III and VI (P<0.05)[13]. Overall tolerability was good. No significant adverse events were recorded apart from mild burning or itching in 5 patients which was transient and resolved with antihistamines and emollients when needed. However, more studies with large number of patients are needed to characterize pimecrolimus cream as a suitable therapeutic option for vitiligo. Pimecrolimus has been shown to be a safe treatment to atopic dermatitis even with recent concerns about carcinogenesis, and its side effect profile is poor [3]. These findings are encouraging for its use in vitiligo treatment, mainly when there is serious concern about local side effects of topical steroids as in periocular and genital areas in children especially when prolonged treatment periods are needed. References
1.
Kovacs SO. Vitiligo. J Am Acad Dermatol. 1998; 38: 647- 66.
PMID: 9591808
2.
Kanwar AJ, Dogra S and Parsad D. Topical tacrolimus for treatment of
childhood vitiligo in Asians. Clin Exp Dermatol, 2004; 29: 589- 92.
PMID: 15550128
3.
Leite RMS and Leite AAC. Two therapeutic challenges: pericocular and genital
vitiligo in children successfully treated with pimecrolimus cream. Int J
Dermatol. 2007; 46: 986- 89.
PMID: 17822508
4.
Hautmann G and Panconesi E. Vitiligo: a psychologically influenced and
influencing disease. Clin Dermatol, 1997; 15: 879- 90.
PMID: 9404691
5.
Njoo MD, Westernhof W, Bos D, et al. The development of guidelines for the
treatment of vitiligo. Arch Dermatol, 1999; 135: 1514- 21.
PMID: 10606057
6.
Norris DA, Kissinger RM, Naughton GM, et al. Evidence of immunologic
mechanisms in human vitiligo. J Invest Dermatol. 1988; 90: 783- 89.
PMID: 3373009
7.
Lan CCE, Chen GS, Chiou M-H, et al. FK 506 promotes melanocyte and
melanoblast growth and creates a favourable milieu for cell migration via
keratinocytes. Br J Dermatol, 2005; 153: 498- 505.
PMID: 16120133
8.
Kemp EH, Waterman EA and Weetman AP. Autoimmune aspects of vitiligo.
Autoimmunity, 2001; 34: 65- 77.
PMID: 11681494
9.
Ongenae K, Van Geel N and Naeyaert J-M. Evidence for autoimmune pathogenesis
of vitiligo. Pigment Cell Res, 2003; 16: 90- 100.
PMID: 12622785
10.
Hegedus L, Heidenheim M, Gervil M , et al. High frequency of thyroid
dysfunction in patients with vitiligo. Acta Derm Venereol, 1994; 74: 120- 3.
PMID: 7911617
11.
Shong YK and Kim JA. Vitiligo in autoimmune thyroid disease. Thyroidology.
1991; 3: 89- 91.
PMID: 1726907
12.
Dawid M, Veensaku M Grassberger M, et al. Efficacy and safety of
pimecrolimus cream 1% in adult patients with vitiligo: Results of a
randomized, double blind vehicle controlled study. JDDG, 2006; 942- 46.
PMID: 17081269
13.
Fai D, Cassano N and Vena GA. Narrow band UVB phototherapy combined with
tacrolimus ointment in vitiligo. JEADV. 2007; 21: 916- 20.
PMID: 17659000
14.
Whitton ME, Ashcroft DM, Barrett CW, et al. Interventions for vitiligo.
Cochrane Data base Syst Rev 2006; 25: CD003263.
15.
Scherschun L, Kim JJ and Lim HW. Narrow-band ultraviolet-B is a useful and
well tolerated treatment for vitiligo. J Am Acad Dermatol, 2001; 44:
999-1003.
PMID: 11369913
16.
Gambichler T, Breuckmann F, Boms S, et al. Narrow-band UVB phototherapy in
skin conditions beyond psoriasis. J Am Acad Dermatol, 2005; 52: 660- 670.
PMID: 15793518
17.
Yu HS, Wu CS, Yu Cl , et al. Helium-neon laser irradiation stimulated
migration and proliferation in melanocytes and induces pigmentation in
segmental type vitiligo. J Invest Dermatol, 2003; 120: 56- 64.
PMID: 12535198
18.
Wellington K and Noble N. Pemicrolimus: A review of its use in atopic
dermatitis. Am J Clin Dermatol 2004; 5: 479- 95.
PMID: 15663345
19.
Eichenfield LF and Beck L. Elidel (pimecrolimus) cream 1%; a non steroidal
topical agent for the treatment of atopic dermatitis. J Allergy Clin Immunol.
2003; 111; 1154- 68.
PMID: 12743593
20.
Silverberg NB, LIN P, Travis L, et al. Tacrolimus ointment promotes
repigmentation of vitiligo in children. J Am Acad Dermatol, 2004; 51: 760-
66.
PMID: 15523355
21.
Luger T and Paul C. Potential new indications of topical calcineurin
inhibitors. Dermatology. 2007; 215 Suppl 1: 45- 54.
PMID: 18174692
22.
Grimes PE, Soriano T and Dytoc M. Topical tacrolimus for repigmentation of
vitiligo. J Am Acad Dermatol 2002; 47: 789- 791.
PMID: 12399778
23.
Coskun B, Saral Y and Turkut D. Topical 0.05% clobetasol propionate versus
1% pimecrolimus ointment in vitiligo. Eur J Dermatol 2005; 15: 88- 91.
PMID: 15757818
24.
Kang HY and Choi YM. FK 506 increases pigmentation and migration of human
melanocytes. Br J Dermatol 2006, 155: 1037- 40.
PMID: 17034537
25.
Hamzavi I, Zain.H, Mc Lean D, et al. Parametric Modeling of Narrow band UVB
phototherapy for vitiligo using a Novel Quantitative Tool. Arch. Dermatol.
2004; 140: 677- 83.
PMID: 15210457
26.
Noursis, M J. Statistical Package for Social Science SPSS base 8.0 for
windows. User's Guide. Chicago, IL: SPSS.1997.
27.
Fricain JC, Sibaud V, Campana F, et al. Mucosal pigmentation after oral
lichen planus treatment with topical tacrolimus. Dermatology. 2005; 210:
229-32.
PMID: 15785053
28.
Hickey JR, Robson A, Barker JN, et al. Does topical tacrolimus induce
lentigines in children with atopic dermatitis? A report of three cases. Br J
Dermatol. 2005; 152: 152- 54.
PMID: 15656817
29.
Luger T and Paul C. Potential new indications of topical calcineurin
inhibitors. Dermatology. 2007; 215 Suppl 1: 45- 54.
PMID: 18174692
30.
Mayoral A, Gonzalez C, Shah NS, et al. Repigmentation of vitiligo with
pimecrolimus cream: A case report. Dermatol, 2003; 207: 322- 23.
PMID: 14571079
31.
Hartmann A, Bröcker EB and Hamm H. Repigmentation of pretibial vitiligo with
calcineurin inhibitors under occlusion. J Dtsch Dermatol Ges. 2008; 6(5):
383- 5.
PMID: 18042249.
32.
Seirafi H, Farnaghi F, Firooz A, et al. Pimecrolimus cream in repigmentation
of vitiligo. Dermatology. 2007; 214(3): 253- 9.
PMID: 17377388
33.
Boone B, Ongenae K, Van Geel N, et al. Topical pimecrolimus in the treatment
of vitiligo. Eur J Dermatol. 2007; 17(1): 55- 61.
PMID: 17324829
34.
Mayoral FA, Vega JM, Stavisky H et al. Retrospective analysis of
pimecrolimus cream 1% for treatment of facial vitiligo. J Drugs Dermatol.
2007 May; 6(5): 517- 21.
PMID: 17679186
35.
Yu HS, Chang KL, Yu CL, et al. Alterations in IL-6, IL-8, GM-CSF, TNFa and
IFN-g release by peripheral mononuclear cells in patients with active
vitiligo. J Invest Dermatol, 1997; 108: 527- 29.
PMID: 9077486© 2008 Egyptian Dermatology Online Journal |




