|
|
Abstract:
Background:
Autoimmune urticaria represents a relatively recent diagnostic advance
since the discovery of histamine releasing autoantibodies against IgE
and IgE receptors. Interleukin-18 (IL-18) is an immuno-regulatory cytokine and has roles in both allergic and
autoimmune disorders.
Aim:
The aim of this work was to
measure IL-18 in patients with chronic urticaria (CU) to find a
correlation between IL-18 levels, clinical disease severity, autologous
serum skin test (Asst) and basophil histamine releasing antibodies.
Patients and methods:
thirty two patients with CU and sixteen healthy control subjects were
included in this study. The disease was classified according to the
number and the size of wheals into mild, moderate and severe. All the
patients and the controls underwent (Asst) to detect histamine releasing
antibodies, basophil histamine release (BHR) assay. IL-18 levels were
measured and (BHR) were assayed in the sera.
Results:
In this study, the mean ages of the patients± standard mean of error (SME)
was 42.6±2.6 years. The number of patients according to the severity of
the disease was, mild=17, moderate=11 and severe=4 patients. The mean ±
SME of IL-18 levels in pg/ml was 251.25 ± 19.69 in patients vs 215.20 ±
28.41 in healthy control with a non significant difference (P=0.29).
When Asst assessment was
done, 50% of patients were scored Asst +ve and 50% were scored Asst -ve.
BHR assays were +ve in (56.25%) of Asst +ve patients and in only
(12.50%) of Asst -ve patients and the mean percentage histamine release
was 15.26 ± 5.6 and 6.10 ± 0.6 in both groups respectively showing a
significant difference, P=0.042. IL-18 levels did not differ
significantly between Asst +ve (243.75 ± 29.93 vs 258.75 ± 26.40 in Asst
-ve patients, P=0.71). When
IL-18 levels were compared in the groups of patients which
were
classified according to Asst and BHR, (Asst +ve, BHR +ve and -ve * Asst-ve,
BHR +ve and -ve), a non significant differences were found, but when its
levels compared with percentage in
histamine release, a significant relation was detected in Asst +ve, BHR
+ve. (r=0.07, value more than 0.001 is
significant).
When patients with CU were grouped according to the severity of the
disease, the highest serum IL-18 levels were detected in patients with
severe disease with a significant +ve correlation in Asst +ve (P=0.04)
and a non significant correlation in Asst -ve (P=0.07).
Conclusion:
IL-18 may play an important role in immune activation in patients with
CU.
Introduction:
Urticaria is a vascular reaction
pattern affecting one fifth of the population, sometime or the other in
their lives. The primary lesions of urticaria are wheals that appear
suddenly as red, itchy, circumscribed areas of dermal oedema, lasting
for few hours and then fade away to reappear in the same or other sites
[1].
Chronic urticaria is defined as recurrence of wheals for at least six
weeks with or without angioedema. In at least 30% of the patients, the
urticaria is chronic, and may severely worsen the quality of life [2].
The basic mechanism for the
urticaria is degranulation of tissue mast cells and blood basophils
(either immunological, non immunological or idiopathic) [3].
Pin pointing to the cause of chronic urticaria may be challenging or
impossible because of the many and varied triggers. In only 25% of the
cases the cause is known [3].
The remainder is labelled
chronic idiopathic urticaria. Autoimmune urticaria represents a
relatively recent diagnostic advance and this condition is now believed
to account for as many as 40% of patients with chronic idiopathic
urticaria [4].
It has been evolved as evidence for histamine releasing autoantibodies
and their relationship to the disease activity has accrued and allowed
the differentiation between chronic autoimmune and chronic idiopathic
urticaria [5,6].
IgG class autoantibodies against
IgE and/or the high affinity IgE receptors have been demonstrated in the
serum of patients with CU [7].
Autologous serum skin test is a screening test for histamine releasing
autoantibodies [5,6].
Thyroid diseases and other autoimmune diseases have been reported to be
associated with CU [5].
Biopsies of lesions of CU reveal a perivascular accumulation of
eosinophils, mast cells, activated CD4 lymphocytes, consisting of Th1
and Th2 subtypes and neutrophils suggesting a role for lymphocyte-mast
cell activation in the patho-mechanism
of CU [8].
Interleukin-18 (IL-18) is an
immuno regulatory cytokine. It is produced by monocytes / macrophages
and dendritic cells in its active form [9].
Keratinocytes, Langerhans
cells, B cells and other epithelial cells throughout the body produce IL
-18. It exerts its effects on the immune system through activation of
the T helper lymphocytes (Th1, Th2) responses depending upon the
cytokine environment [10].
Thus,
IL-18 may play a role in
autoimmune and allergic disorders characterized by Th1 and Th2 responses
respectively [11].
The biological effects of IL-18 are mainly due to the production of IFNγ
[12].
Few studies have been undertaken
to explore the role of IL-18 in (CU) [13].
In this study, serum IL - 18 will be measured and its possible relation
to histamine releasing autoantibodies and the severity of the disease
will be studied.
Patients and Methods :
Thirty two adult patients (20
females and 12 males) with chronic urticaria were chosen randomly from
the Allergy and Immunity and Skin Departments of Zagazeg University
Hospitals, during the period from January to June 2008. Their ages
ranged from (16-65 years). The diagnosis was dependant on the recurrence
of wheals with or without angioedema for more than 6 weeks. In all the
patients, the known causes of chronic or recurrent urticarias were ruled
out by careful history taking and by the proper investigations as CBC,
ESR, ANA, ALT, AST, kidney function tests, and
urine and stool analysis.
Patients with physical urticarias or other chronic inflammatory diseases
including atopic dermatitis were also excluded from the study.
Sixteen gender matched and age matched healthy
subjects were used as a control group. This group included 4 males and
12 females with age ranging from 16 to 62 years.
Patients and control subjects
signed informed consents and patients
agreed to discontinue systemic
antihistamines 5 days prior to the date of testing. Disease activity was
estimated according to the number of wheals present at the time when the
serum samples for thirty two patients were collected [14]:
1 to 10 small (< 3cm in diameter) wheals = grade 1 or mild; 10 to 50
small wheals or 1 to 10 large wheals = grade 2 or moderate; > 50 small
wheals or > 10 large wheals = grade 3 or severe; and virtually covered
with wheals = very severe or grade 4.
All the patients and control
subjects underwent the following tests:-
·
Autologous serum skin test (Asst): This test was performed by
an intradermal injection of 0.05ml of fresh autologous serum and read
after 30 minutes. We used a normal saline solution ( 0.9% NaCl sol)
injected intradermally as a negative control and histamine solution
1mg/ml for the skin prick test as a positive control. Wheals 1.5 mm
greater than that produced by the normal saline were considered positive
[5].
·
Basophil Histamine Release (BHR) assay: In this test, leucocyte
suspensions from three normal blood donors were separated by dextran and
stimulated to histamine release on challenge with an optimum dose
(10µg/ml) of goat poly colonal anti-human 1gE and was showing a 30% net
release. (Sigma Chemical Co. St. Louis, Mo, USA). Histamine
concentration was measured by an automated fluorimetric method. A 5% net
release cut off value was used. One assay per serum was performed [15]
to detect histamine releasing activity.
·
Serum IL-18 concentration was measured by using a sandwich
enzyme immunoassay with sensitivity of 12.5pg/ml (MBL. Medical and
Biological Laboratories, Nagoya, Japan), according to Asero et al. 2007.
Statistical Analysis: -
Mann - Whitney U test, Kruskal -
Wallis test with Dunn's multiple comparisons test, Fisher's exact test
and Spearmann rank correlation test were used in the study. P values <
0.05 were considered significant. All the results were expressed in the
form of mean ± SME.
Results:
In this study, thirty two
patients with CU (their mean ages was 42.6 ±2.6 years) and sixteen
healthy age and sex matched control subjects (their mean ages was 43.5 ±
3.8 years) were enrolled. The patients grades according to the severity
of the disease were mild=17, moderate=11 and severe=4 patients. IL-18
concentrations in patients with CU was 251.25 ±19.69 Pg /ml and its
levels in normal control subjects 215.20 ± 28.41 Pg /ml showed a non
significant difference as in Table 1.
|
Number |
Mean ± SME |
Range |
Median |
t
p |
|
Cases |
251.25±19.69 |
75-525 |
217.5 |
1.05 0.29 |
|
No =32 |
|
Control |
215.20±28.41 |
70-400 |
220 |
Non significant |
|
No =16 |
SME:
standard mean of error.
Table 1 :Comparison between serum
IL-18 concentrations(pg/ml) in patients with chronic urticaria and
control .
Regarding the results of Asst,
16 patients (50%) were scored +ve for Asst and 16 patients (50%) were
scored Asst - ve. IL-18 concentration did not differ significantly as in
Table 2 between Asst +ve (243.75 ± 29.93) Pg /ml and Asst -ve
(258.75 ± 26.40) groups. BHR was +v in 9 (56.25%), Asst +ve and in only
2(12.50%) Asst - ve patients with CU.
| |
Number of cases |
Percent |
Mean ± SME |
Range |
Median |
t p |
|
Asst positive |
16 |
50% |
243.75±29.95 |
75-525 |
197.5 |
0.31 0.71 |
|
Asst negative |
16 |
50% |
258.75±26.40 |
125-485 |
225 |
Non significant |
Asst: Autologous
serum skin test, SME: standard mean of error.
Table 2: Comparison between serum
IL-18 concentrations(pg/ml) in both Asst positive and Asst negative
patients with chronic urticaria.
The mean ± SME percentage histamine release in both groups were
15.56±5.60 and 6.10±0.60 respectively showing a significant difference
as in Table 3.
| |
Number of cases |
Mean ± SME |
Range |
Median |
t p |
|
BHR +ve in Asst positive |
9 |
15.56±5.60 |
7-23.80 |
16.6 |
2.33 0.042 |
|
BHR -ve in Asst negative |
2 |
6.10±0.60 |
5.70-6.30 |
6.1 |
Significant |
Asst: autologous serum skin test,
SME: standard mean of error.
Table 3: Percentage Histamine release in both Asst positive and Asst
negative patients with chronic urticaria
When percentages histamine release in Asst +ve patients with +ve BHR
were compared with serum IL-18 concentrations, a significant relation
was present (r=0.77, value more than 0.001 was significant). When IL-18
levels compared in patients with CU grouped according to Asst and BHR
(Asst +ve, BHR +ve and -ve * Asst -ve, BHR +ve and -ve), a non
significant differences were found as in Table 4, but when its
levels correlated with the disease severity, the highest serum IL-18
levels were detected in patients with severe disease with a significant
+ve correlation in Asst +ve and a non significant correlation in Asst -ve
group as in Fig.1
| |
Number of cases |
Percent % |
Mean ± SME |
Range |
Median |
t p |
Asst +ve
BHR+ve |
9 |
56.25 |
241.74±36.93 |
75-405 |
195 |
0.07 0.93 |
Asst +ve
BHR -ve |
7 |
43.75 |
246.42±52.76 |
140-525 |
200 |
Non significant |
Asst -ve
BHR -ve |
14 |
87.5 |
265.33±29.93 |
125-485 |
245 |
0.4 0.5 |
Asst -ve
BHR+ve |
2 |
12.5 |
212.51±2.47 |
210-215 |
212.5 |
Non significant |
BHR: basophil histamine release,
SME: standard mean of error.
Table 4: Comparison of serum IL-18 concentrations according to Asst
and BHR positive and negative in patients with chronic urticaria.
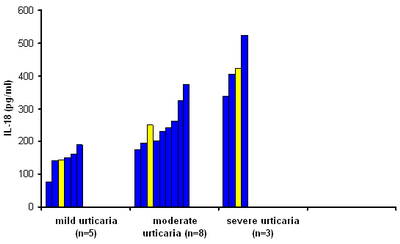
|
Fig 1a:
Serum IL-18 levels in ASST-positive patients
classified according to clinical severity score.
Statistical analysis by Kruskal-Wallis Test showed
that there was a significant difference between the
3 groups (P= 0.04). Post tests were performed by
Dunn's multiple comparison tests. The yellow color
represents the mean values, the blue color
represents the original values. |
|
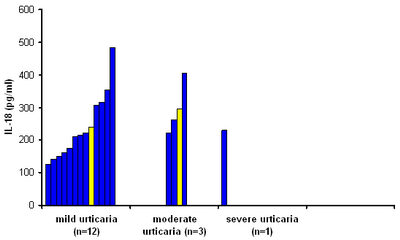
|
Fig 1b:
Serum
IL-18 levels in ASST-negative patients classified
according to clinical severity scor. No significant
difference was found between the patient groups
(P=0.07). The yellow color represents the mean
values, the blue color represents the original
values. |
|
Discussion:
Urticaria is one of the most
common dermatological problems that cause frustration to the patients
and physicians. The impact on
the quality of life that CU causes to the patients is so distressing,
especially sleep disturbance, and can be difficult to treat [16].
CU is without a clear etiology in the majority of cases, but the basic
mechanism is the degranulation of mast cells and basophils with release
of histamine and vasoactive mediators [3].
Interleukin-18 was measured in
the serum of patients with CU in this study. Its level did not differ
significantly between patients and healthy control subjects, and did not
differ between Asst +ve and Asst -ve groups. This is in agreement with
the previous results [13].
In the present study, when the
patients with CU were grouped according to the severity of the disease,
there was a positive correlation between serum IL-18 concentrations and
the severity of the disease in Asst +ve patients and showed a tendency
to correlate positively with BHR. These data suggested that IL-18 has
been proposed to play a role in mast cell degranulation and histamine
release as a direct factor. This may be through the production of IL-4
and increase in IgE level and histamine release by basophils [17].
In Asst -ve group, this
correlation was not found .Other mechanisms than the direct effects of
IL-18 were suggested [8,21].
Up to 50% of patients with CU
included in this study were Asst +ve due to the presence of histamine
releasing autoantibodies that is in agreement with the previous studies
[4,5].
In only 9 out of 16 Asst +ve patients, BHR was +ve, detecting the
presence of functioning histamine releasing autoantibodies [18,19].
In this group antibody mediated mast cell activation is involved [20].
It has been suggested that
lymphocyte- mast cell activation may be a possible mechanism in the
remaining of patients as evidenced by the presence of a perivascular
accumulation of an infiltrate
rich in CD4 lymphocytes, consisting of Th1 and Th2 cells in the biopsy
of lesions from CU patients [8],
or it may be due to activation of the extrinsic pathway of coagulation,
with the production of thrombin. Thrombin is able to stimulate mast cell
degranulation and C5a that enhances histamine release induced by FcЄR
autoantibodies [21,22].
In conclusion:
IL-18
may be involved in chronic urticaria and its level correlates with the
disease severity in Asst +ve patients and shows a tendency to correlate
with in vitro histamine release. This suggests that the immune system is
strongly activated by IL-18 which may have some role as a direct factor.
Recommendations:
As IL-18 may have some role in CU, in the future it can be inhibited by
caspase-1 inhibitors [23]
and IL-18 binding protein which is able to bind IL-18 with a high
affinity and to inhibit IL-18 induced IFN-γ and IL-18 production [24]
to treat some cases of autoimmune urticaria and other auto immune
diseases,
a
subject that needs more researches.
References
1. Sharma B, Gera
MB,Tivari I TH Chronic urticaria: Expanding the autoimmune kaleidoscope, MJAFI
2004; 60: 372.
2. Clive EH, Ruth A, Malcom W et al. Chronic urticaria .J Am.
Acad. Dermatol. 2002; 46: 645.
3. Ring J, Brockow K, Ollert M et al. Antihistamines In Urticaria. Clinical Exp
Allergy 1999; 29 (suppl. 1): 31.
4. Hein R .Chronic urticaria: impact of allergic inflammation. Allergy 2002; 571
(75): 19.
5. Sabroe R A, Gratten CA, Francis DM et al. The autologous serum skin test: A
screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol
1999; 140: 446.
6. Gratten CE, The urticaria spectrum recognition of clinical pattern can help
management. Clin Exp Dermatol 2004; 29: 217.
7. Ferrer M, Kint JP, Kaplan AP.Comparative studies of functional and binding
assays for IgG anti FCЄR1α (α subunit) in chronic urticaria. J Allergy Clin
Immunol 1998; 101: 672.
8. Ying S, Kikuchi Y, Meng Q et al. TH1/TH2 cytokines and inflammatory cells in
skin biopsy specimens from patients with chronic idiopathic urticaria:
comparison with the allergen induced late phase cutaneous reaction. J Allergy
Clin Immunol 2002; 109: 694.
9. Nakanishi K, Yoshimoto T, Tsutsui H et al. Interleukin-18 regulates bothTh1
and Th2 responses. Ann Rev Immunol 2001; 19: 423.
10. Boraschi D and Dinarello C. IL-18 in autoimmunity: review. Eur Cytokine Netw
2006; 17(4): 224.
11. Izakovicova Holla L. Interleukin in asthma and other allergies. Clin Exp
Allergy 2003; 33: 1023.
12. Nakamura K, Okamura H, Wada M et al. Endotoxin -induced serum factor that
stimulates gumma interferone production. Infect. Immunol 1989; 37: 590.
13. Tedeschi M, Lorini C. Suli and Asero R. Serum IL-18 in patients with chronic
ordinary urticaria association with disease activity. Clin Exp Dermatol 2007;
32: 568
14. Sabroe RA, Fiebiger E, Francis DM et al. Classification of anti -FCЄR1
and anti IgE autoantibodies in chronic idiopathic urticaria and correlation with
disease severity. J Allergy Clin Immunol 2002; 110: 492.
15. Asero R, Tedeschi A , Lorini M et al. Chronic urticaria. Novel clinical and
serological aspects. Clin Exp Allergy 2001; 31: 1105.
16. O'Donnell BF, Lawlor F, Simpson J et al. The impact of chronic urticaria in
the quality of life indices. Br J Dermatol 1997; 136: 197.
17. Yoshimoto T, Tsutusi H, Tominaga Ket al. IL-18, although antiallergic when
administered with IL-12, stimulates IL-4 and histamine release by basophils.
Proc Natl Acad Sci USA 1999; 96: 13962.
18. Greaves MW. Chronic Urticaria. J Allergy Clin Immunol 2000; 105: 664.
19. Sabroe RA and Greaves MW. Chronic idiopathic urticaria with functional
autoantibodies: 12 years on. Br J Dermatol 2006; 154: 813.
20. Boguniewicz M. The autoimmune nature of chronic urticaria.
Allergy Asthma Proc 2008; 29: 433.
21. Asero R, Riboldi P, Tedeschi A et al.
Severe Chronic Urticaria: A disease at a crossroad between autoimmunity and
coagulation. Autoimmunity Review 2007; 7: 71.
22. Asero R, Tedeschi A, Riboldi P et al. Severe chronic urticaria is associated
with elevated plasma levels of D-dimer. Allergy 2008; 63:176.
23. Linton SD. Caspase inhibitor, A pharmaceutical industry
perspective.
Curr Top Med Chem 2005; 5: 1697.
24. Novick D, Kim SH, Fantuzzi Get al.Interleukin -18 binding protein a novel
modulator of Th1 cytokine response. Immunity 1999; 445: 338.
© 2009 Egyptian Dermatology Online Journal
|


