|
|
Abstract
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a member of the tumor necrosis factor superfamily, and has
been implicated in the regulation of various physiological and pathological
immune responses. The present study aims to study TRAIL expression in skin
lesions and non-lesional skin of patients with atopic dermatitis (AD) as
well as in lichen simplex chronicus and in normal control skin. The study
has been conducted on 49 patients with atopic dermatitis, 3 cases were
acute, 15 cases were subacute, 28 cases were chronic and 3 cases were lichen
simplex chronicus. Two skin biopsies were obtained from all patients;
one from lesional skin and the other from the apparently normal non-lesional skin. Skin biopsies
were
also obtained from 3 normal volunteers. Immunohistochemical staining
technique was performed to demonstrate TRAIL expression in skin samples of
patients and controls. In the cells of the dermal infiltrate the expression
of TRAIL was the higher in acute lesions (80 cells ± 4/H.P.F.) than subacute
(25cells±5/H.P.F.) and chronic lesions (20 cells ± 2/H.P.F.) and the
differences were highly significant (p=<0.0001 for both). The expression in
non-lesional skin (50 cells ± 5/H.P.F.) was significantly lower than acute
lesions and lichen simplex ((p=<0.0001 and =0.045 respectively),
significantly higher than subacute and chronic lesions (p=0.037 and =0.014
respectively) but was not statistically different from normal controls. In
conclusion, the expression of TRAIL in skin of patients with AD
differs according to the stage of the disease. To better understand its
functions, we suggest that TRAIL expression studies should be performed in
other diseases and controlled following pharmacological treatment.
Introduction
Atopic dermatitis (AD) is characterized by chronic or
relapsing pruritic eczematous lesions with a typical morphology and
distribution.[1]
The pathogenesis of AD is not completely understood and involves a complex
series of interactions between resident and infiltrating cells orchestrated
by proinflammatory cytokines and chemokines.[2]
In the dermis of AD lesions, there is a marked perivascular T cell
infiltrate,[3,4]
in which both CD4+ve and CD8+ve T cells are present.[5]
The infiltrating T cells express pro-inflammatory cytokines, in particular,
interleukin (IL)-5 and IL-13.[6,7]
In chronic lesions, IFN-γ-producing cells have also been described.[8,9]
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a member of the tumor necrosis factor (TNF) superfamily,
and has been implicated in the regulation of various physiological and
pathological immune responses.[10]
This might, at least partially, be because of its wide expression among
cells of the immune system, including activated T cells,[11,12,13,14]
B cells,[11,15]
monocytes,[16,17,18]
dendritic cells,[19]
natural killer cells,[14,20,21]
and neutrophils.[18,22,23]
The TRAIL system consists of the ligand (TRAIL) and
five different cellular receptors (TRAIL-R1 to TRAIL-R5).[24]
TRAIL-R1 and TRAIL-R2 are death receptors that have the ability to initiate
the apoptosis- signalling cascade after ligation, whereas TRAIL-R3 and
TRAIL-R4 are decoy receptors lack this ability and are actually reported to
prevent extensive apoptosis in cells and tissues expressing both TRAIL and
the death receptors, TRAIL-R1 and -R2. Osteoprotegerin is a soluble receptor
for TRAIL and may also act as a soluble decoy receptor. The balance of the
expression levels between the death receptors and decoy receptors is an
important factor determining the apoptotic effect of TRAIL.[25,26]
TRAIL originally received considerable attention
following the observation that it selectively induced apoptosis in cancer
but not in normal cells.[27,28]
In addition to its therapeutic potential, TRAIL might act as a natural
guardian eliminating transformed cells at an early stage.[29]
However, recent work suggested that TRAIL also has several physiological
functions that are not limited to the killing of transformed cells. For
instance, TRAIL has been shown to induce apoptosis in several primary cells,
such as hepatocytes, [30,31]
HIV-activated T cells, [32]
plasma cells, [33]
immature dendritic cells, [34]
and neutrophils.[22,23]
Regulators of TRAIL expression have been reported such TNF-α, which is a
down-regulator of TRAIL expression, whereas IFN-γ up-regulates the
expression of TRAIL. [35]
The present study aims to study tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) expression in acute,
subacute and chronic skin lesions as well as in non-lesional skin of
patients with atopic dermatitis as well as in normal control skin samples
for more understanding of the pathogenesis of this disease.
Materials and Methods
The present study has been conducted on 49 patients
with atopic dermatitis, attending the Dermatology outpatient clinic of Al-Minya
University Hospital. Twenty-four patients (49%) were males and 25 (51%) were
females. The age of patients ranged from 1 year to 35 years with a mean age
and Standard Deviation (SD) of 9.1 ± 7.3 years. All patients satisfied the
UK Working Party's refinement of the diagnostic criteria of the Hanifin and
Rajka for atopic dermatitis. [36]
Of all patients included three cases (6.1%) were acute, 15 cases (30.6%)
were subacute, 28 cases (57.1%) were chronic and 3 cases (6.1%) were lichen
simplex chronicus. Patients received no topical or systemic treatment for at
least 1 month before biopsy taking.
Skin Biopsies
Skin biopsies were obtained from all patients using
sterile 4 mm punches after taking informed consents. Two biopsies were
taken; one from lesional skin and the other from the
apparently normal non-lesional skin. Each biopsy was immediately fixed in
10% formalin, embedded in a paraffin block and sectioned into 5 µm thick
sections. These sections were utilized for routine haematoxylin and eosin
(H&E) as well as for immunohistochemical staining. Skin biopsies were also
obtained from 3 normal control persons and have been subjected to the same
embedding and staining procedures as for patient biopsies.
Immunohistochemistry
Immunohistochemical staining technique was performed to demonstrate TRAIL
expression in skin samples of patients and controls. A ready-to-use
detection system (lab EnVision + System, Peroxidase (DAB), lab vision®, Cat#
TP-015-HD) was used. The primary antibody was the monoclonal mouse
anti-human TRAIL /TNFSF10 antibody (R&D system Corporation®, Cat# 375). It
was used in a dilution of 25µg/ml. The expression of TRAIL in the cells of
the dermal infiltrate was evaluated according to the number of positively
stained cells/H.P.F. (Table 1).
|
Score |
Number of positive cells/
H.P.F. |
|
0 |
No positively stained cells |
|
1 |
1- 25 cells |
|
2 |
26 – 50 cells |
|
3 |
51 – 75 cells |
|
4 |
75-100 cells |
|
5 |
> 100 cells |
Table 1: Scoring
system for quantifying TRAIL expression in the cells of the dermal
infiltrate.
Statistical analysis
Data were collected and tabulated using Excel
Software. Statistical analysis was done using SPSS (version 11.0). The
numerical data was expressed as mean ± standard deviation (SD). T-student
test was used to compare numerical values. The (t-test) values were
expressed in terms of p-value. P value was considered significant when it is
< 0.05
and highly significant when it is < 0.001.
Results
TRAIL was noticed to be expressed mainly in the cells
of the dermal infiltrate. In addition, some degree of expression was also
noticed in vessel wall, endothelial cells, fibroblasts as well as
periappendageal.
TRAIL expression in dermal infiltrate
Lesional skin
(Table2; Fig.2-4)
The expression of TRAIL was found to be much higher in the cells of the
dermal infiltrate in acute than subacute or chronic cases. In acute cases,
the mean TRAIL expression was found to be 80 cells ± 4/H.P.F. (score 4)
(Fig.2). This expression was noticed to
decline to 25 cells ± 5/H.P.F (score 1-2) in subacute cases (Fig.3).
This decline is statistically highly significant (p=<0.0001). The mean TRAIL
expression also declined to reach 20 cells ± 2/H.P.F (score 1) in chronic
cases (Fig.4). This decline is also statistically highly significant
(p=<0.0001).
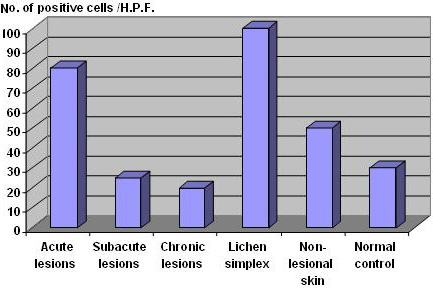 | Fig 1:
TRAIL expression in the dermal infiltrate of acute, subacute and
chronic lesions of AD, lichen simplex chronicus as well as in
non-lesional skin and normal controls. |
|
|
|
|
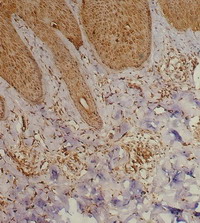
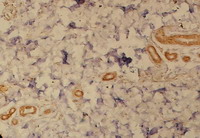
| Fig 2: Acute atopic skin lesion showing marked
TRAIL expression in the dermal infiltrate as well as in
epidermal cells, periappandageal, perivascular and
endothelial cells (Immunoperoxidase; X200). |
|
|
|
|
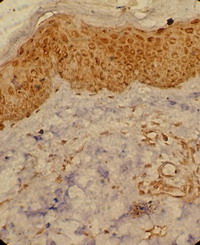
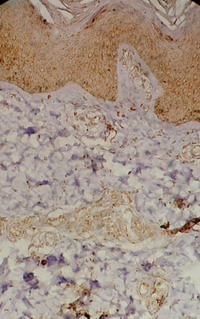
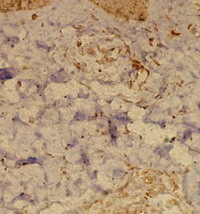
| Fig 3: Subacute atopic skin lesion showing
moderate TRAIL expression in the dermal infiltrate as well
as in epidermal cells, periappandageal, perivascular and
endothelial cells (Immunoperoxidase; X200). |
|
|
|
|
| Fig 4: Chronic atopic skin lesion showing mild
TRAIL expression in the dermal infiltrate as well as in
epidermal cells, periappandageal, perivascular and
endothelial cells (Immunoperoxidase; X200). |
|
Lichen simplex
(Table2; Fig.5)
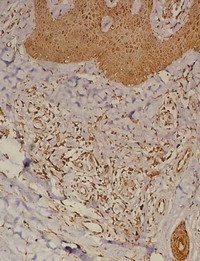
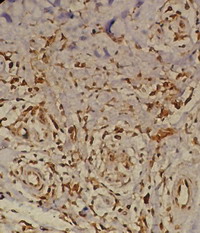
Biopsies obtained from patients with lichen simplex
showed high expression of TRAIL (100 cells ± 24/H.P.F; score 4-5) in the
dermal infiltrate (Fig.5). Although this expression was not
statistically different from that observed in acute lesions (p=0.2), it was
much higher than that noticed in subacute and chronic skin lesions as well
as non-lesional skin and this difference is statistically highly significant
(p=<0.0001).
|
|
|
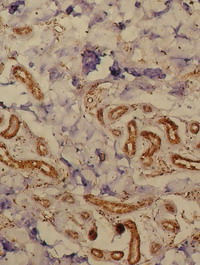
| Fig 5: Lichen simplex lesion showing TRAIL
expression in the dermal infiltrate as well as in epidermal
cells, periappandageal, perivascular, endothelial cells and
fibroblasts (Immunoperoxidase; X100). |
|
Non-lesional skin (Table2; Fig.6)
Biopsies obtained from non-lesional skin of patients
with AD showed moderate level (50 cells ± 5/ H.P.F; score 3) of TRAIL
expression in the dermal infiltrate (Fig.6). The level of expression
was lower than that observed in the acute lesions of AD and this difference
was statistically highly significant (p=<0.0001).
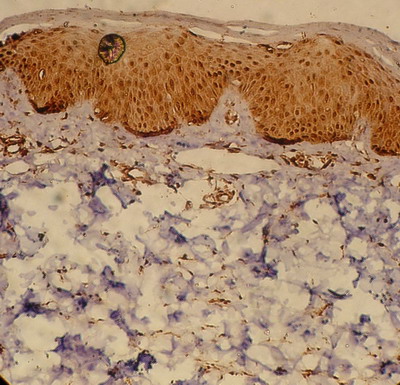 | Fig
6:
Non-lesional skin of an atopic patient showing moderate TRAIL
expression in the epidermis with mild expression in the dermal
inflammatory infiltrate (Immunoperoxidase; X200). |
|
Normal control
(Table2; Fig.7)
Biopsies obtained from normal control subjects also showed moderate level
(30 cells ± 5/ H.P.F.; score 3) of the TRAIL expression in dermal infiltrate
(Fig.7). The expression was lower than that observed in acute lesions
of AD as well as in non-lesional skin and this difference was statistically
highly significant (p=0.0001 for both). To the contrary, TRAIL expression in
the skin samples of normal controls was higher than that observed in chronic
skin lesions of AD and this difference was statistically highly significant
(p=0.0001). However, when compared with that observed in subacute lesions,
the expression was not statistically different (p=0.12).
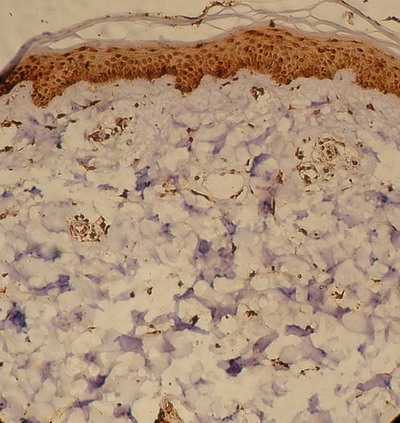 | Fig
7: Skin from a normal control subject showing marked TRAIL
expression in the epidermis with mild expression in the dermal cells
(Immunoperoxidase; X200). |
|
|
|
Acute (N=3) |
Subacute (N=15) |
Chronic (N=31) |
Lichen Simplex
(N=3) |
Non-lesional skin
(N=10) |
Normal skin (N=3) |
|
Mean number of
TRAIL +ve cells/H.P.F. in dermal infiltrate |
80±4 |
25±5 |
20±2 |
100±24 |
50±5 |
30±4 |
Table 2: TRAIL
expression in dermal infiltrate of lesional and non-lesional skin of
patients with AD, lichen simplex chronicus and in normal controls.
Discussion
In spite of the recent progress in the field, the
physiological role of TRAIL remains to be determined. [39]
TRAIL has been shown to be critical in the regulation of the immune
response. It induces apoptosis in dendritic cells, regulating adaptive
immune response,[19]
and it mediates apoptosis in neutrophils, [18,22,23]
suggesting that it also controls innate immune responses. In addition, TRAIL
also exerts anti-inflammatory activities. [22,23,30,31,32,33,34,37,38,39,40].
An understanding of the immunologic basis of AD may have important
implications for clinical management of the disease. [41]
The present study aims to study TRAIL expression in lesional as well as in
non-lesional skin of patients with atopic dermatitis and comparing it with
normal control samples for more understanding of the pathogenesis and
immunologic basis of this disease.
In the present study, the expression of TRAIL in the
cells of the dermal infiltrate was observed to be significantly higher in
acute than in subacute (p=<0.0001) or chronic skin lesion (p=<0.0001) of
patients with AD. In addition, the level of expression in acute lesions was
also higher than that observed in non-lesional skin (p=<0.0001) of patients
with AD as well as in normal control skin (p=0.0001). These findings are
consistent with previous reports. TRAIL-positive mononuclear cells were
present in greater numbers in lesional skin of patients with AD compared
with non-lesional skin and normal controls.[42]
Our results show that TRAIL is expressed in a high
level at an early stage of inflammation in acute skin lesions of AD. After
subsidence of the acute phase, the level of TRAIL expression starts to decline to
be almost close to the expression level found in normal skin. This suggests
that TRAIL, with its immune regulatory and anti-inflammatory effects, is
expressed in a high level at an early stage of inflammation in an attempt to
control inflammation and minimize tissue damage. Once the acute phase
resolved and the acute inflammation started to subside, the level of TRAIL
gradually declines to its normal level found in normal skin. This view may
be supported by the finding that the proportion of TRAIL-expressing CD8
cells, with their cytotoxic/ suppressive role, are two folds higher than CD4
cells in lesional skin of AD patients.[39]
It was suggested that TRAIL exerts its anti-inflammatory activities through
the induction of apoptosis in inflammatory cells,[22,23,30,31,32,33,34]
blocking of the cell cycle, [37]
activation of inhibitory phosphatases,[38]
and increasing the expression of IL-1Ra.[39]
The latter has a crucial role in the prevention of massive tissue damage
during inflammatory responses. [40]
The results of the present study also show that the
non-lesional skin of patients with AD expresses a moderate level of TRAIL in
cells of the dermal inflammatory infiltrate. TRAIL expression in cells of
the dermal inflammatory infiltrate in non-lesional skin was significantly
lower that observed in acute lesions (p==<0.0001) but significantly more
than that observed in subacute and chronic skin lesions as well as in normal
control skin. This observation suggests that the apparently normal skin of
patients with AD harbour some immunohistochemical abnormalities regarding
TRAIL expression with more than normal expression in dermal inflammatory
infiltrate. Thus we suggest adding a new finding to the list of
abnormalities previously reported [41]
in the apparently normal skin of patients with AD.
In patients with lichen simplex, skin biopsies showed
expression of TRAIL in the cells of the dermal infiltrate, which was not
significantly different from that observed in acute skin lesions of AD
(p=0.2). However, the level of expression was significantly higher than that
seen in subacute lesions (p=<0.0001), chronic lesions (p=<0.0001), non-lesional
skin (p=<0.0001) as well as normal control subjects (p=0.007). To the best
of our knowledge, TRAIL expression was not previously studied in the lesions
of lichen simplex chronicus. We suggest that the persistence of inflammation
in such persistent and chronic lesions may be responsible for the continued
expression of TRAIL by the infiltrating cells as well as by the local tissue
in an attempt to terminate such chronic inflammatory process. In addition,
we suggest considering this highly significant difference (p=<0.0001) in
TRAIL expression in lesions of lichen simplex chronicus as a point of
immunohistochemical differentiation between this type of eczema and the
ordinary chronic skin lesions of AD.
In conclusion,
the expression of TRAIL in skin of patients with AD differs according to the
stage of the disease, being the highest in the acute stage then declines
thereafter. Non-lesional skin of patients with AD is abnormal regarding
TRAIL expression with more than normal expression in dermal inflammatory
infiltrate. The lower than normal TRAIL expression in the ordinary chronic
skin lesions of AD helps to differentiate them from those of lichen simplex
chronicus. To better understand its functions, we suggest that TRAIL
expression studies should be performed in other diseases and controlled
following pharmacological treatment.
References1. Leung DYM, Bieber T. Atopic dermatitis. Lancet 2003; 361: 151- 160.
2. Boguniewicz M, Leung YMD. Atopic dermatitis. J Allergy Clin Immunol 2006; 117: S475- 80.
3. Braathen LR, Fo¨rre O, Natvig JB, Eeg-Larsen T. Predominance of T lymphocytes in the dermal infiltrate of atopic dermatitis. Br J Dermatol 1979; 100: 511- 519.
4. Leung DYM, Bhan AK, Schneeberger EE, Geha RS. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J Allergy Clin Immunol 1983; 71: 47- 56.
5. Akdis CA, Akdis M, Simon D, et al. T cells and T cell-derived cytokines as pathogenic factors in the nonallergic form of atopic dermatitis. J Invest Dermatol 1999; 113: 628- 634.
6. Hamid Q, Boguniewicz M, Leung DYM. Differential in situ cytokine expression in acute versus chronic atopic dermatitis. J Clin Invest 1994; 94: 870- 876.
7. Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DYM. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol 1996; 98: 225- 231.
8. Grewe M, Gyufko K, Schopf E, Krutmann J. Lesional expression of interferon gamma in atopic eczema. Lancet 1994; 343: 25- 26.
9. Trautmann A, Akdis M, Kleemann D, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000; 106: 25- 35.
10. Kimberley FC, Screaton GR. Following a TRAIL: Update on a ligand and its five receptors. Cell Res 2004; 14: 359- 372.
11. Mariani SM, Krammer PH. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur J Immunol 1998; 28: 1492- 1498.
12. Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 Tcell killing of target cells. J Immunol 1998; 161: 2195- 2200.
13. Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med 1999; 189: 1451- 1460.
14. Mirandola P, Ponti C, Gobbi G, et al. Activated human NK and CD8T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood 2004; 104: 2418- 2424.
15. Kemp TJ, Moore JM, Griffith TS. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation. J Immunol 2004; 173: 892- 899.
16. Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte- mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med 1999; 189: 1343- 1354.
17. Halaas O, Liabakk NB, Vik R, Beninati C, Henneke P, Sundan A, Espevik T. Monocytes stimulated with group B streptococci or interferons release tumour necrosis factor-related apoptosis-inducing ligand. Scand J Immunol 2004; 60: 74- 81.
18. Tecchio C, Huber V, Scapini P, et al. IFNalpha-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood 2004; 103: 3837- 3844.
19. Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Exp Med 1999; 190: 1155- 1164.
20. Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med 1998; 188: 2375- 2380.
21. Kayagaki N, Yamaguchi N, Nakayama M, et al. Expression and function of TNFrelated apoptosis-inducing ligand on murine activated NK cells. J Immunol 1999; 163: 1906- 1913.
22. Renshaw SA, Parmar JS, Singleton V, et al. Acceleration of human neutrophil apoptosis by TRAIL. J Immunol 2003; 170: 1027- 1033.
23. Matsuyama W, Yamamoto M, Higashimoto I, et al. TNF-related apoptosis-inducing ligand is involved in neutropenia of systemic lupus erythematosus. Blood 2004; 104: 184- 191.
24. Daniel PT, Wieder T, Sturm I & Schulze-Osthoff K. The kiss of death: Promises and failures of death receptors and ligands in cancer therapy. Leukemia 2001; 15: 1022- 1032.
25. Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998; 10: 559- 63.
26. Ashkenazi A, Dixit V. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 1999; 11: 255- 60.
27. Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104: 155- 162.
28. Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157- 163.
29. Stander S, Schwarz T. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is expressed in normal skin and cutaneous inflammatory diseases, but not in chronically UV-exposed skin and non-melanoma skin cancer. Am J Dermatopathol. 2005; 27(2): 116-21.
30. Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 2000; 6: 564- 567.
31. Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest 2004; 113: 58- 64.
32. Miura Y, Misawa N, Maeda N, et al. Critical contribution of tumor necrosis factor related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4 T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J Exp Med 2001; 193: 651- 660.
33. Ursini-Siegel J, Zhang W, Altmeyer A, Hatada EN, Do RK, Yagita H, Chen-Kiang S. TRAIL/Apo-2 ligand induces primary plasma cell apoptosis. J Immunol 2002; 169: 4739- 4744.
34. Leverkus M, Walczak H, McLellan A, Fries HW, Terbeck G, Brocker EB, Kampgen E. Maturation of dendritic cells leads to up-regulation of cellular FLICEinhibitory protein and concomitant down-regulation of death ligand-mediated apoptosis. Blood 2000; 96: 2628- 2631.
35. Kamohara H, Matsuyama W, Shimozato O, Abe K, Galligan C, Hashimoto S, Matsushima K, Yoshimura T. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology 2004; 111(2): 186- 194.
36. Kefei, K. A. M. Poster; Susan, T. N.; Seth, R. S. and Kevin, D. C. (): Atopic dermatitis. Papulosquamous and eczematous dermatoses. In: Dermatology. Bolognia JL, Jorizzo JL, Rapini RP (eds), Mosby. 2006; P199.
37. Song K, Chen Y, Goke R, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med 2000; 191: 1095- 1103.
38. Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med 2002; 8: 61- 67.
39. Vassina, E, Martin L, Shida Y, Lasse RB, Hans-Uwe S, Dagmar S. Increased expression and a potential anti-Inflammatory role of TRAIL in AD. J Invest Dermatol 2005; 125: 746 -752.
40. Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor. Rev 2002; 13: 323- 340.
41. Leung DYM, Soter NA. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol 2001; 44: S1- 12.
42. Warnnissorn P, Nakao A, Suto H, Ushio H, Yamaguchi N, Yagita H. Tumor necrosis factor-related apoptosis-inducing ligand expression in atopic dermatitis. Br J Dermatol 2003; 148: 829- 831
© 2009 Egyptian Dermatology Online Journal |











