|
|
Abstract
Background: Leprosy commonly affects the cranial nerves
predominantly the 5th (trigeminal nerve) and the 7th (facial nerve). Lepra
reactions are risk factors for cranial nerve involvement.
Objective: To study the frequency and pattern of cranial nerve
involvement in leprosy and to find its relation with facial patch.
Patients and methods: The present clinical study was undertaken on
100 consecutive leprosy patients to find out the involvement of cranial
nerves in leprosy and to study the relationship between cranial nerve
involvement and leprosy patch/patches on facial skin.
Results: Cranial nerve involvement was detected in 22 patients on
clinical grounds; 7 Borderline Tuberculoid (BT), 6 Lepromatous Leprosy (LL),
6 Borderline Lepromatous (BL), 1 Pure Neuritic (PN), 1 Tuberculoid
Tuberculoid (TT) and 1 Borderline Borderline (BB). The most commonly
involved cranial nerves were the facial and trigeminal, seen in 9% each;
followed by the olfactory in 6% and the auditory in 3%. Most cases with
facial and trigeminal nerve involvement were of BT leprosy types while the
majority with olfactory and auditory nerve involvement was of the
lepromatous leprosy type (BL, LL). The association between lagophthalmos of
recent origin, type 1 lepra reaction and significant facial patch was
statistically significant. Introduction
Leprosy is the most common cause of treatable peripheral neuropathy in India
and probably also in the world because of the large number of affected
individuals perhaps closely matched by diabetic neuropathy [14].
A cardinal sign is sensory loss which always precedes paralysis in all types
of leprosy [17].
Leprous neuropathy is characterized by involvement of superficial nerve
trunks in areas such as ulnar, radial cutaneous, median, common peroneal,
supra orbital and greater auricular which are cool and liable to trauma [10].
Any cranial nerve can be affected predominantly the 5th and the 7th
[15].
The zygomatic branch of facial nerve which supplies the orbicularis oculi
muscle is the most frequently affected [16].
Appearance of type 1 reaction puts a patient at risk of nerve damage and
secondary deformities [6].
Early recognition and medical treatment of early nerve damage with steroids
may result in full restoration of nerve function. The presence of
significant facial patch around eyes or over malar region together with type
1 lepra reaction is a severe risk factor for the development of
lagophthalmos and paralysis of other facial muscles [6].
Hypaesthesia and anaesthesia are most often observed in maxillary division
of the 5th nerve [16].
In most cases of facial nerve involvement in leprosy, there is sensory
impairment or hypopigmented and hypoanesthetic patch as well in the
territory of trigeminal nerve especially over the maxillary branch. It
becomes conceivable that leprosy infection entering the malar skin through
sensory fibres progresses in such a way that it involves the peripheral
motor branches of facial nerve in the area [5].
Sensorineural hearing loss is more in lepromatous leprosy with ENL (Erythema
Nodosum Leprosum) reaction [2].
Involvement of nasal mucosa is greatest in lepromatous leprosy. The present
study was undertaken to assess the frequency and pattern of involvement of
cranial nerves, to characterize the type of leprosy that may be associated
with damage of cranial nerves and to unravel some characteristics that were
not explored in the past. Material and methods
One hundred consecutive leprosy patients diagnosed on
the basis of skin lesions, nerve involvement, slit skin smear examination
and histopathological examination enrolled / attending the urban leprosy
centre were screened for cranial nerve involvement irrespective of the type,
duration or treatment status of the disease. Detailed history and complete
clinical examination of each patient was performed with respect to age, sex,
duration of disease, number, morphology and distribution of skin lesions
including facial patch if any, nerve involvement with particular emphasis on
cranial nerve involvement, duration of cranial nerve involvement, type of
leprosy and treatment status. Ophthalmological and ENT consultations were
conducted wherever required. Patients with complaints like nasal stuffiness,
deviated nasal septum, intranasal adhesions and scarring in nasal mucosa (i),
cataract (ii), history of head injury (iii, iv, vi, vii), acute/chronic ear
discharge/drug intake such as aminoglycosides, salicylates, antiepileptics,
tranquillizers, diuretics or family history of hearing loss (viii), history
of diabetes, hypertension, renal impairment, anaemia etc were excluded
because related cranial nerve couldn't be assessed accurately in them.
Individual cranial nerves were tested clinically for sensory, motor and
special functions. No specialized laboratory or electrophysiological tests
were conducted (except audiometery for confirmation of sensironeural hearing
loss). The recorded data was tabulated and analysed using chi square test.Results and observations
The study material included 76 males and 24 females in
the age ranging from 10 to 70 years. Twenty- two patients had cranial nerve
involvement; 9 (18%) of 50 PB and 13 (26%) of 50 MB cases. Most of the
patients with cranial nerve involvement were in age group 21-40 (50%) with a
mean age of 42 years and male female ratio of 3.4:1.
The majority of cases were of BT (32%) followed by LL (25%), BL (20%), TT
(9%), PN (9%) and BB (5%). Thirty percent of BL patients had cranial nerve
involvement followed by 24% LL and 22% BT.
Cranial nerve
involved |
Type of leprosy |
Total |
|
PN |
TT |
BT |
BB |
BL |
LL |
|
Facial |
- |
- |
4 |
1 |
2 |
2 |
9 |
|
Trigeminal |
1 |
1 |
4 |
- |
2 |
1 |
9 |
|
Olfactory |
- |
- |
1 |
- |
2 |
3 |
6 |
|
Auditory |
- |
- |
1 |
- |
1 |
1 |
3 |
|
Total |
1 |
1 |
10 |
1 |
7 |
7 |
27 |
Table 1: Cranial
nerve involvement across the spectrum.
Facial and trigeminal nerves were the most commonly involved cranial nerves.
On analysis of spectrum of leprosy, 4 out of 9 cases of facial nerve
involvement were seen in BT, 2 each in BL and LL and 1 in BB. Trigeminal
nerve involvement was seen in 4 BT patients, 2 each of BL and LL patients
and 1 each of PN and TT patients. Involvement of the olfactory nerve was
seen in 6 patients, 3 of them had LL, 2 BL and 1 BT. Auditory nerve
affection was seen in 3 patients only, 1 each of BT, BL and LL (Table 1).
|
No of cranial nerves involved |
Type of leprosy |
Total |
|
PN |
TT |
BT |
BB |
BL |
LL |
|
1 |
1 |
1 |
5 |
1 |
5 |
5 |
18 |
|
2 |
- |
|
1 |
- |
1 |
1 |
3 |
|
>2 |
- |
|
1 |
- |
- |
- |
1 |
|
Total |
1 |
1 |
7 |
1 |
6 |
6 |
22 |
Table 2:
Number of cranial nerves involved and type of
leprosy.
One cranial nerve involvement was seen in 18 out of 22 patients, two in 3
(13.64%) patients and three in 1 (4.54%) patient. Overall, 27 cranial nerves
were affected in 22 patients (Table 2).
|
Muscle |
MB |
PB |
Total |
%age |
|
Frontalis |
Unilateral |
1 |
1 |
2 |
22.22 |
|
Bilateral |
1 |
1 |
2 |
22.22 |
|
No involvement |
3 |
2 |
5 |
55.66 |
|
Orbicularis oris |
Unilateral |
1 |
2 |
3 |
33.33 |
|
Bilateral |
2 |
0 |
2 |
22.22 |
|
No involvement |
2 |
2 |
4 |
44.44 |
Table 3:
Affected muscles other than orbicularis oculi
muscle in 9 lagophthalmos patients (5 MB, 4 PB).
Weakness of the orbicularis oculi muscle (lagophthalmos)
was seen in all the 9 (100%) patients with facial nerve involvement.
Weakness of the frontalis muscle was found in 4 (44.44%) patients and that
of orbicularis oris muscle in 5 (55.55%) patients. Involvement of buccinator
and platysma muscles or loss of taste sensation was not detected in any
patient (Table 3, Fig 1).
Five patients had some form of unilateral facial palsy and 4 had bilateral
affection. In LL and BL cases there was more symmetrical pattern of
paralysis (50%) than in BT/BT+ cases (25%) which was not significant
(p>0.50).
|
|
No of Patients with recent lagophthalmos |
Total |
|
Significant patches |
4 |
12 |
|
Other/No patches |
3 |
88 |
|
Total |
7 |
100 |
Table 4:
Relationship between recent lagophthalmos and
facial patches.
The facial patches were classified as significant in
12 patients and other patches in 25 patients. Significant patch is pale and
flat or red and raised lesion (in type 1 reaction), located around the eye
or in the malar region at least 3 cm in diameter (Table 4).
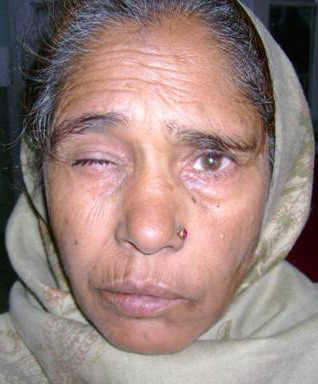
Lagophthalmos of recent origin was present in 4 (33.33) of 12 patients with
significant facial patches (Fig 2). Patients with facial patch had a
statistically significant higher frequency of involvement of facial nerve (p
<0.02).
|
|
No of patients with recent lagophthalmos |
Total |
|
With type 1 reaction |
4 |
24 |
|
Without type 1 reaction |
3 |
76 |
|
Total |
7 |
100 |
Table 5:
Relationship between recent lagophthalmos and
type 1 reaction
Type
1 reaction was present or apparent during treatment in 24 patients.
Lagophthalmos of recent origin (of <1 year duration) was found in 7 patients out
of total of 9 patients with lagophthalmos and in 4 patients with type 1
reaction. The association between lagophthalmos of recent origin and type 1
lepra reaction was statistically significant (p <0.02) (Table 5)
|
|
Recent lagophthalmos |
No of patches |
|
Significant patch with type 1 reaction |
4 |
8 |
|
Significant patch without type 1 reaction |
0 |
4 |
|
Total |
4 |
12 |
Table 6:
Relationship between recent lagophthalmos,
significant patches and type 1 reaction.
Lagophthalmos was present in 4 (50%) of 8 patients who
had both significant facial patches and type 1 reaction. The lagophthalmos
was without exception at the same side as patch and in case of large patches
covering the whole face, it was often bilateral. Altogether, 4 (57.14%) of 7
patients with recent lagophthalmos had significant red and raised patches in
type 1 reaction (Table 6).
Trigeminal nerve affection was seen as loss of corneal
reflex in all 9 (100%) patients, hypaesthesia in maxillary area in 4
(44.44%) patients, hypaesthesia in ophthalmic area in 2 patients and
hypaesthesia/anesthesia in 2 patients in mandibular area. Five cases had
sensory loss in the form of hypaesthesia of face and anesthesia in 1
patient. Motor weakness was not seen in any case.
Impairment of olfaction was found in 6 (6%) cases as
asymptomatic hyposmia (1 BT, 2 BL, 3 LL). Changes in nasal mucosa were not
seen in any case.
Sensorineural hearing loss was detected in 3 (1 BT,
1 BL, 1 LL) cases only and hearing impairment was discovered on
tuning fork testing and confirmed by audiometery. Hearing impairment was
bilateral in 1 (BL) and unilateral in 2 other cases. Conductive hearing loss
was not detected in any patient. Evaluation of vestibular system by clinical
testing did not reveal any abnormality.
 | Fig 1:
Left paresis of frontalis, orbicularis oculi &
orbicularis oris with deviation of angle of mouth towards right side. |
|
 |
Fig 2:
Significant facial patch in a patient with recent lagophthalmos. |
|
Discussion
The hallmark of leprosy is invasion and inflammation
of nerves which are present in all stages of different varieties of the
disease. Although its overall prevalence is decreasing, leprosy continues to
be a major cause of peripheral neuropathy worldwide. Although leprosy
usually affects the superficial nerves, yet any nerve in the body including
the cranial nerves can be affected. It is important to note that changes
secondary to cranial nerve impairment can be as trivial as loss of smell
which seems to be of minor importance to the patient to as severe as
disfigurement and disabilities such as blindness and deafness.
Twenty- two (22%) of the 100 consecutive patients in
present study had cranial nerve involvement. The 5th and 7th
nerves were
most frequently affected. Thappa et al (2004) and Kumar et al (2006)
reported incidences of 22% and 18% with facial nerve involvement of 10% and
9.8% respectively. Incidence of cranial nerve involvement was more in
multibacillary leprosy (26%) than paucibacillary leprosy (18%) which was not
statistically significant (p>0.50). This was attributed to probably longer
duration of disease in multibacillary patients.
The majority of our patients with facial nerve
involvement had BT leprosy. Various studies [11,15,16,19]
found majority of their patients with facial palsy in BT. Lagophthalmos was
seen in all our 9 patients (100%) with facial nerve involvement. This could
be because of the superficial location of the zygomatic branch and large,
red, facial patches in malar region or around eye in 4 of our patients with
lagophthalmos.
Out of 9 patients, 3 (1 PB, 2 MB) had developed
bilateral facial palsy. Lubbers et al (1994) stated that in LL and BL
leprosy cases, there is symmetrical pattern of paralysis and in BT/BT+
cases, pattern is asymmetrical. This observation was also made in our study,
but was not significant (p>0.50) possibly due to lower number of cases. We
did not find weakness of buccinator or platysma in our study comparable to
other studies [3,4,19].
It is well known that the appearance of type 1
reaction puts a patient at risk of nerve damage and secondary deformities [6].
The relationship between lagophthalmos of recent origin and type 1 reaction
was statistically significant (p>0.02) in the present study. Facial nerve
involvement in leprosy occurs only when there are coexisting skin lesions on
face and there is centripetal involvement of branches of facial nerve [1].
The relationship between lagophthalmos and facial patch was statistically
significant (p<0.02). Both these observations are comparable to other
studies [6,19].
The association of facial nerve involvement, facial
patch and type 1 reaction was also highlighted by Hogeweg et al (1991). A
majority of their patients (85%) with recent lagophthalmos had significant
patch over the malar region or around the eye on the same side as nerve
damage together with clinical signs of type 1 reaction. In our study 4 out
of 7 patients (57.14%) with recent lagophthalmos had significant patch over
malar region or periorbital area together with type 1 reaction. Leprosy
infection entering the malar skin through sensory fibres progress in such a
way as to involve the peripheral motor branches of facial nerve in the area
[5].
Thappa et al (2004) and Kumar et al (2006) found
trigeminal nerve involvement in 7% and 8% of their patients respectively.
Reichart et al (1982) found trigeminal nerve affection in the form of
hypaesthesia and anesthesia of face and no motor weakness was detected in
any of their patients. The predominant manifestations were loss of corneal
reflex in all our 9 patients, similar to that observed by Konuncu et al
(1994) and Ramadan et al (2001).
Reichart et al (1982) found the majority of patients
with trigeminal nerve affection in BT leprosy. The pattern of hypaesthesia
and anesthesia in our study were comparable to that of facial nerve lesions
since frontal and maxillary divisions were also often affected. As in facial
paralysis, most cases of hypaesthesia and anesthesia were seen in BT.
Olfactory nerve involvement was seen as hyposmia in 6
patients (6%), majority (3 cases) belonged to LL. Kaur et al (1979) found
anosmia in 4% patients all with LL and Thappa et al (2004) observed
olfactory loss in 9% cases. This could be attributed to longer duration of
disease in lepromatous leprosy.
Auditory nerve involvement (cochlear part) was seen in
3 (3%) of our patients. Most other studies [2,9,13,15,18]
observed higher frequency of cochlear nerve impairment whereas Thappa et al
(2004) and Kumar et al (2006) detected 3% and 2% cochlear nerve involvement
respectively. However, it should be noted that audiometeric testing was not
performed in our patients that could be limitation in assessing the exact
involvement of auditory nerve. Koyuncu et al (1994) observed 11.1% of their
patients with vestibular dysfunction which is contrary to other studies [2,8,13]
including ours.
Among 22 patients with cranial nerve involvement, only
those with facial nerve involvement knew the duration of involvement of
nerves. The duration of facial nerve involvement ranged from 3 months to 12
years with mean duration of 1.91 years. Seven out of 9 patients had
lagophthalmos of less than one year duration which was comparable to Hogeweg
et al (1991) who studied only those patients with history of recent
lagophthalmos of less than one year duration. Other patients were not aware
of duration of their disabilities.
Conclusion:
One should thoroughly examine the cranial nerve
functions in every case of leprosy as this commonly affects cranial nerves.
All leprosy patients, especially those at increased risk (significant facial
patch in periorbital region, lepra reactions) should be monitored from the
outset of the disease in order to detect nerve damage early and to prevent
permanent loss of function.
References1. Antia NH, Divekar SC, Dastur DK. The facial Nerve in leprosy. Clinical and operative aspects. Int J Lepr. 1966; 34: 103-17
2. Awasthi S, Singh G, Dutta R, et al. Auriculovestibular nerve involvement in leprosy. Indian J Leprosy. 1990; 6
3. Bruce M, Richard, Peter R Corry. Cervical branch of facial nerve in leprosy. Int J Lepr other Mycobact Dis.1997; 65:170-177
4. Crawford CL and Hobbs MJ. Why is the cervical branch of facial nerve not enlarged in leprosy? J Anathes. 1994; 184: 188
5. Dastur D.K. Pathology and pathogenesis of predilective sites of nerve damage in leprous neuritis. Nerves in the arm and the face. Neurosurg Rev. 1983; 6: 139-152
6. Hogeweg M, Udaya Kiran K, Sujai Suneetha. Significance of facial patch & type 1 reaction for development of facial nerve damage in leprosy. A retrospective study among 1226 paucibacillary patients. Lepra Review 1991; 62: 143-149
7. Kaur S, Malik SK, Kumar B, et al. Respiratory system involvement in leprosy. Int J Lepr, 1979; 47: 48
8. Kokhar DK, Gupta DV, Chauhan S, et al. Study of brain stem auditory evoked potentials and visual evoked potentials in leprosy. Int J Lepr other Mycobact Dis, 1997; 65: 157-165
9. Koyuncu M, Celik O, Ozturk A, et al. Audiovestibular system, fifth and seventh cranial nerve involvement in leprosy. Indian J Leprosy, 1994; 66: 421-428
10. Kumar Sudhir, Alexander Mathew, Gnanamuthu Chandan. Cranial nerve involvement in patients with leprous neuropathy. Neurology India, 2006; 54: 283-285
11. Lemieux L, Cherian TA, Richard B. The stapedial reflex as a topographical marker of proximal involvement of the facial nerve in leprosy. A pilot study. Lepr Rev, 1999; 70: 324-332
12. Lubbers WJ, Schipper A, Hogeweg M, et al. Paralysis of facial muscles in leprosy patients with lagophthalmos. Int J Lepr other Mycobact Dis, 1994; 62: 220-224
13. Mann SBS, Kumar B, Yande, et al. Eighth nerve evaluation in leprosy. Indian J Leprosy. 1987; 59: 20-25
14. Paul JT, North-Wilhelm K, Higdon GA, et al. Multiple cranial neuropathies associated with leprosy. South Med J, 1994; 87: 937-940
15. Ramadan W, Mourad B, Fadel W, et al. Clinical, electrophysiologucal and immunopathological study of peripheral nerves in Hansen's disease Lepr Rev, 2001; 72: 35-49
16. Reichart PA, Srisuwan S, Metah D. Lesions of the facial trigeminal nerve in leprosy. Int J Oral Surg, 1982; 11: 14-20
17. Schuring AG and Istre, Hansen's disease and hearing. Arch otolaryngol, 1969; 89: 478-481
18. Singh TR, Aggarwal SK, Bajaj AK, et-al. Evaluation of auriculovestibular status in leprosy. Indian J Leprosy. 1984; 56: 24-29
19. Thapa DM, Gopinath DV, Jaishanker TJ. A clinical study of the involvement of cranial nerves in leprosy. Indian J Leprosy, 2004; 76: 1-9
© 2009 Egyptian Dermatology Online Journal |


