|
|
Abstract
Background: Atopic
dermatitis (AD) is a chronic, relapsing, pruritic and inflammatory skin
disease. Several treatments are available for this condition including
pimecrolimus.
Aim of Work: To study
the histopathologic, histometric and immuno-histochemical
(IH) changes in lesional skin of AD patients following topical pimecrolimus
1% cream.
Subjects and Methods:
The study included 10 patients with AD, aged between 2.5 to 11 years. Skin
biopsies from affected areas were obtained before and after treatment.
Biopsy sections were utilized for routine H&E as well as for IH staining for
CD markers 3, 4, 8, 20 and 68 and tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL).
Results:
Post-treatment biopsies showed insignificant (p=0.06) histopathologic
changes such as mild decrease in the degree of spongiosis, acanthosis,
infiltrate and mean epidermal thickness. However, immunohistochemical
changes were striking. The number of CD3, CD4, CD8 and CD68 positive cells
in the dermal infiltrate showed a significant decline (p=0.04) after
treatment. In addition, the number of TRAIL positive cells in the dermal
infiltrate showed a highly significant decline (p=<0.0001) after treatment.
Conclusion:
Pimecrolimus exerts its therapeutic effects in AD through modulation of the
immunological network underling AD lesions, in particular, through
inhibiting lymphocyte function as well as reducing the number of
TRAIL-positive inflammatory cells.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, highly
pruritic, and inflammatory skin disease [1].
It is caused by complex interaction of many different genes & multiple
environmental factors affecting their expression [2].
The pathogenesis of AD is not completely understood and involves a complex
series of interactions between resident and infiltrating cells orchestrated
by pro-inflammatory cytokines and chemokines [3].
In the dermis of AD lesions, there is a marked perivascular infiltrate in
which both CD4+ and CD8+ T cells are present .The majority of these cells
are of the CD45 Ro+ memory /effector phenotype and express the selective
skin-homing receptor, cutaneous lymphocyte -associated antigen (CLA) [4,5].
Several treatments are available for this condition
including the macrolide immunomodulator pimecrolimus. Pimecrolimus is a safe
and effective treatment in reducing the severity of AD symptoms in children
and adults. It affects mainly T lymphocytes by interacting with a
cyclophilin-like cytoplasmic protein, and this complex in turn inhibits
calcineurin, [6]
a molecule required for initiation of cytokine gene transcription. This
results in inhibition of gene transcription of multiple cytokines with a
considerable importance in inflammation pathway (mainly IL-2, IL-4, and
IL-5). [7]
The present study aims to study the histopathologic,
histometric and
immuno-histochemical (IH) changes that occur in
lesional skin of patients with AD following topical treatment using
pimecrolimus 1% cream, for better understanding of the mechanism of action
of this medication.
Subjects and Methods
The present study has been conducted on 10 patients
with atopic dermatitis, attending the Dermatology outpatient clinic of
Al-Minya University Hospital. Eight patients (80%) were males and two (20%)
were females, their age ranged from 2.5 to 11years with a mean and standard
deviation (SD) of 8.5 +
3.2. All patients satisfied the UK Working Party's
refinement of the diagnostic criteria of the Hanifin and Rajka for atopic
dermatitis [8].
Patients received no topical or systemic treatment for at least 1 month
before being enrolled in the study. Pimecrolimus 1% cream was applied
to skin lesions once daily till
apparent complete clinical clearance.
Skin Biopsies
Skin biopsies from treated areas were obtained before
treatment and after apparent complete clinical clearance, usually after 3
weeks, using sterile 4 mm punches after taking informed consents. Each
biopsy was immediately fixed in 10% formalin, embedded in a paraffin block
and sectioned into 5 µm thick sections. These sections were utilized for
routine hematoxylin and eosin (H&E) as well as for IH staining.
Histometry
The thickness of the epidermis was studied in both
pre- and post-treatment skin biopsies. These histological measurements (histometry)
were carried out on standard H&E stained sections. The distances between the
outermost surface of the epidermis, excluding the horny layer, and the dermo-epidermal
junction were measured at different points throughout the section using an
ocular micrometer. The mean of these measurements was taken as
representative of the mean epidermal thickness (MET).
Immunohistochemistry (IH)
IH staining techniques were used to demonstrate some CD markers for T and
B-lymphocytes and histiocytes a well as to demonstrate tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) expression in skin samples
before and after treatment. A ready-to-use detection system (lab EnVision +
System, Peroxidase (DAB), lab vision®, Cat# TP-015-HD) was used. Primary
antibodies used to demonstrate the different CD markers and their dilutions
are shown in table 1. The primary antibody for TRAIL was the
monoclonal mouse anti-human TRAIL /TNFSF10 antibody (R&D system
Corporation®, Cat# 375). It was used in a dilution of 25µg/ml.
|
CD marker |
Antibody |
Dilution |
Company& Cat# number |
|
CD3 ( Pan T lymphocyte marker) |
Mouse monoclonal anti-human CD3 |
01:50 |
Bio Care Medical®,
Cat# CM110C |
|
CD4 (T-helper lymphocyte marker) |
Mouse monoclonal anti-human CD4
antibody |
ready-to-use |
Lab vision Corporation®,
Cat# MS-1528 |
|
CD8 (T- suppressor/cytotoxic
lymphocyte marker) |
Mouse monoclonal anti-human CD8
antibody |
ready-to-use |
Lab vision Corporation®,
Cat# RM-9116 |
|
CD20 (B lymphocyte marker) |
Mouse monoclonal clone L26
anti-human CD20 antibody |
0.1805556 |
Daco Cytomation®,
Cat# M 0755 |
|
CD68 (Histiocyte marker) |
Mouse monoclonal anti-human CD68
antibody Ab-3 (KP1) |
01:30 |
NeoMarkers®,
Cat# MS-397-P |
Table 1: List of the 1ry antibodies, dilutions and commercial
sources for CD markers used in the study. The expression of CD markers in the dermal infiltrate was evaluated according to the system previously described [9] as shown in
table 2. The expression of TRAIL in the cells of the dermal infiltrate was evaluated according to the number of positively stained cells/H.P.F. as shown in
table 3.
|
Number of positive
cells/H.P.F. |
Score |
|
No staining |
0 (-) |
|
Staining < 25% |
1 (+) |
|
26 – 50 % |
2 (++) |
|
51 – 75 % |
3 (+++) |
|
> 75 % |
4 (++++) |
Table 2: Scoring of CD markers expression in dermal cellular
infiltrate.
|
Number of positive cells/
H.P.F. |
Score |
|
No positively stained cells |
0 |
|
1- 25 cells |
1 |
|
26 – 50 cells |
2 |
|
51 – 75 cells |
3 |
|
75-100 cells |
4 |
|
> 100 cells |
5 |
Table 3: Scoring system for quantifying TRAIL expression in the cells
of the dermal infiltrate. Statistical analysis Data were collected and tabulated using Excel Software. Statistical analysis was done using SPSS (version 11.0). The numerical data was expressed as mean ± SD. Student-t test was used to compare numerical values. The (t-test) values were expressed in terms of p-value. P value was considered significant when it is
< 0.05 and highly significant when it is < 0.001. Results
Histopathologic changes Biopsies taken 3 weeks after treatment with pimecrolimus cream 1% showed only minimal histopathologic changes in the form of mild decrease in the degree of spongiosis, acanthosis, infiltrate and mean epidermal thickness. The latter reduced from a mean of 145μm±30 before treatment to mean of 115μm ±5, however, this difference is statistically not significant (p=0.06)
(Fig.1a, b).
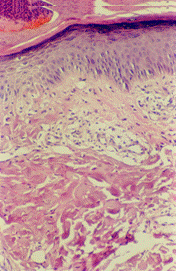 | Fig 1a:
Atopic lesion before treatment with pimecrolimus 1% cream (H&E;
X100). |
|
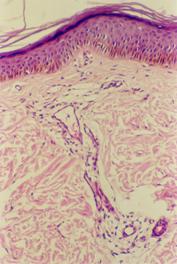 | Fig 1b:
Atopic skin lesion three weeks after treatment with pimecrolimus
1% cream. Only minimal histopathologic changes in the form of
mild decrease in the degree of spongiosis, acanthosis,
infiltrate and epidermal thickness are seen after treatment
(H&E; X100). |
|
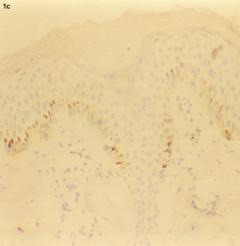 | Fig 1c:
Atopic skin lesion showing negative staining for CD20 in the dermal
infiltrate (H&E, X100). |
|
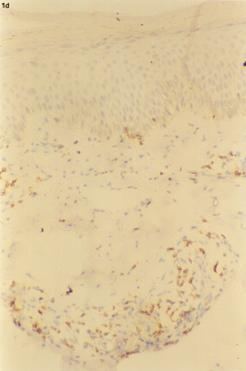 | Fig 1d:
Atopic skin lesion showing positive staining for CD3 in the
dermal infiltrate (H&E: X100). |
|
IH stainingIH staining techniques were performed to study the expression of some CD markers as well as to evaluate TRAIL expression before and after treatment. CD markers: The markers CD3, CD4, CD8 and CD68 showed positive staining in all pre-treatment biopsies. In post-treatment biopsies the expression of all of these CD markers had declined. This difference is statistically significant (p=0.04). The marker CD20 was negative in lesional skin and remained as such even after three weeks of topical pimecrolimus treatment
(Table 4; Fig.1-5).
|
|
Before treatment |
After
treatment |
|
CD marker |
Number of positive
cells/H.P.F. |
Score |
Number of positive
cells/H.P.F. |
Score |
|
CD3 |
40±2 |
++ |
11±1 |
+ |
|
CD4 |
20±3 |
+ |
7±2 |
+ |
|
CD8 |
18±3 |
+ |
8±2 |
+ |
|
CD20 |
- |
- |
- |
- |
|
CD68 |
60±2 |
+++ |
30±3 |
++ |
p=0.04
Table 4: Score of CD markers
expression before and after treatment with pimecrolimus 1% cream.
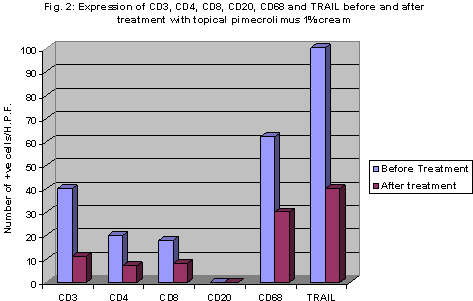 | Fig
2: Expression of CD3, CD4, CD8, CD20, CD68 and TRAIL before and
after treatment with topical pimecrolimus 1% cream. |
|
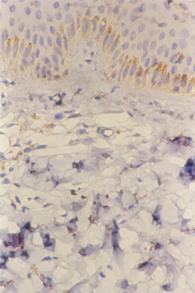 | Fig
3a: CD 4 staining in atopic skin lesion before treatment (Immunoperoxidase
technique; X200). |
|
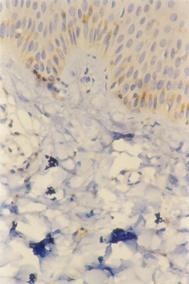 | Fig
3b: Atopic skin lesion three weeks after treatment with
pimecrolimus 1% cream, showing decrease in the number of CD4+ve
cells in the dermal infiltrate after treatment (Immunoperoxidase
technique; X400). |
|
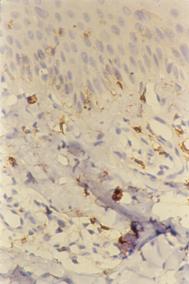 | Fig
4a: CD8 staining in atopic skin lesion before treatment (Immunoperoxidase
technique; X200). |
|
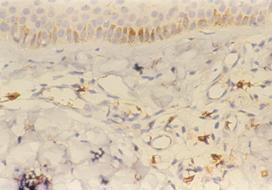 | Fig
4b: Atopic skin lesion three weeks after treatment with
pimecrolimus 1% cream, showing decrease in the number of CD8+ve
cells in the dermal infiltrate after treatment (Immunoperoxidase
technique; X200). |
|
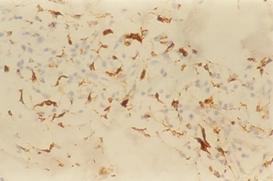 | Fig
5a: Atopic skin lesion showing positive staining for CD68 in the
dermal infiltrate (H&E, A: X200). |
|
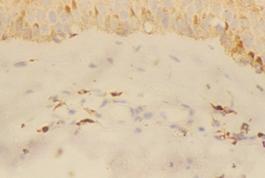 | Fig
5b: Atopic skin lesion showing diminished staining for CD68
in the dermal infiltrate 3 weeks after treatment with
pimecrolimus (H&E, A: X200) |
|
TRAIL expression: TRAIL expression was found to show marked decrease in biopsies obtained 3 weeks after treatment as compared to pre-treatment biopsies. The expression decreased from a mean of 100±10 cells/H.P.F before treatment to a mean of 40±4 cells/H.P.F after treatment. This difference is statistically highly significant (p=<0.0001)
(Table 5; Fig.2 and Fig.6a-d).
|
|
Before treatment |
After treatment |
|
Number of positively-stained cells/H.P.F. |
100±10 cells/H.P.F. |
40±4 cells/H.P.F. |
|
Score of positively-stained cells |
4 |
2 |
|
Intensity of staining |
3 |
2 |
|
Mean score |
3.5 |
2 |
p=<0.0001
Table 5: Score of TRAIL expression before and after treatment with
pimecrolimus 1% cream.
|
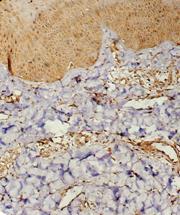
|
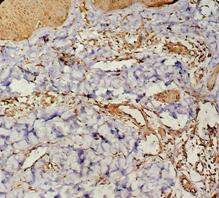
|
| Fig 6a&b: TRAIL +ve cells in the dermal
infiltrate of atopic skin lesion before treatment (Immunoperoxidase
technique; X200). |
|
|
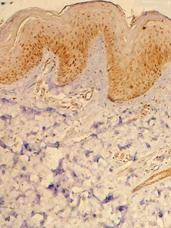
|
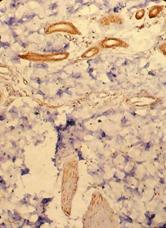
|
| Fig 6c&d: Atopic skin lesion three weeks after
treatment with pimecrolimus 1% cream, showing decrease in
the number of TRAIL +ve cells in the dermal infiltrate after
treatment (Immunoperoxidase technique; X200). |
|
Discussion
Atopic dermatitis is one of the most common inflammatory skin disorders [10]. Two main concepts evolved to explain its pathogenesis: excessive T-cell activation in response to an antigen and hyperstimulation of T cells by atopic Langerhans cells [11,12,13]. Topical corticosteroids treatment is a mainstay in treatment of AD; although long-term use is limited by its side effects [14]. However, the evolving understanding of the pathogenesis of AD has allowed researchers to target specific steps in the inflammatory cascade and this has driven the development of more effective, steroid-free therapies to treat AD [13], such as the calcineurin inhibitor pimecrolimus, that has proven to be a novel option for AD treatment [15,16]. This work aims to study the histopathologic, histometric and IH changes that occur in AD skin lesions following topical pimecrolimus 1% cream. In the present study post-treatment biopsies has shown only minimal histopathologic changes in the form of mild decrease in the degree of spongiosis, acanthosis and inflammatory infiltrate. In addition, post-treatment biopsies has shown minimal, but insignificant, decrease in the mean epidermal thickness (p=0.06). Our observation suggests that topical pimecrolimus 1% cream does not cause epidermal atrophy, a common problem with long-term topical corticosteroid treatment [14]. However, since post-treatment biopsies in our study were obtained after a relatively short treatment period (usually 3 weeks), therefore post-treatment biopsies after longer treatment periods will be required to confirm our observation. Although histopathologic changes were minimal, IH changes were striking. The number of CD3, CD4, CD8 and CD68 positive cells among cells of the dermal infiltrate showed a significant decline (p=0.04) after 3 weeks of topical pimecrolimus treatment
(Table 4). This suggests that this therapy results in a significant reduction in the number of T-helper cells, suppressor/cytotoxic T cells and histiocytes in treated skin of patients with AD. We suggest that this decline in the number of these inflammatory cells may be an indicator of subsiding of the acute inflammatory phase of AD under the effect of this treatment. Pimecrolimus, being targeting T cells and mast cells, inhibits the production and release of cytokines and other inflammatory mediators as well as the expression of signals essential for the activation of inflammatory T lymphocytes [17] and thus exerts a beneficial effect in AD.
Furthermore, the number of TRAIL positive cells in the
dermal infiltrate has shown a highly significant decline (p=<0.0001) from a
mean of 100 + 10 cells/H.P.F. to a mean of 40
+ 4 cells/H.P.F. after 3 weeks of topical
pimecrolimus treatment (Table 5). TRAIL is a member of the TNF
superfamily, and has been implicated in the regulation of various
physiological and pathological immune responses [18].
This might be because of its wide expression among cells of the immune
system, including activated T cells [19],
B cells [20,21],
monocytes [22],
dendritic cells [23],
natural killer cells [19],
and neutrophils [22].
Since TRAIL-positive mononuclear cells are present in greater numbers in
lesional skin of patients with AD compared with non-lesional skin and normal
controls [24],
a decline in the number of TRAIL positive inflammatory cells following
topical pimecrolimus treatment again may be an indicator of subsiding of the
acute inflammatory phase of the disease under the effect of this treatment.
To the best of our knowledge TRAIL expression was not previously evaluated
in patients with AD following treatment with pimecrolimus.
In conclusion, the results of the present study
suggests that pimecrolimus exerts its therapeutic effects in AD through
modulation of the immunological network that underlies AD lesions, in
particular, through inhibiting lymphocyte function as well as through
reducing the number of TRAIL-expressing inflammatory cells. Further studies
on a larger number of patients are recommended to confirm this latter
observation.
References
1. Leung DYM, Atopic dermatitis: New insights and opportunities for therapeutic intervention, J Allergy Clin Immunol, 2000; 105: 860- 876.
2. Leung DYM, Bieber T, Atopic dermatitis, Lancet, 2003; 361: 151- 159.
3. Boguniewicz M, Leung YMD, Atopic dermatitis, J Allergy Clin Immunol, 2006; 117: S475- 480.
4. Akdis CA, Akdis M, Simon D et al, T cells and T cell-derived cytokines as pathogenic factors in the non-allergic form of atopic dermatitis, J Invest Dermatol, 1999; 113: 628- 634.
5. Trautmann A, Akdis M, Br?cker E, et al, New insights into the role of T cells in atopic dermatitis and allergic contact dermatitis, Tred Immunol, 2001; 22(10): 530-532.
6. Nghiem P, Pearson G, Langley RG, Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis, J Am. Acad. Dermatol, 2002; 46, 228- 241.
7. Galli E, Cicconi R, Rossi P, et al, Atopic dermatitis: molecular mechanisms, clinical aspects and new therapeutical approaches, Curr Mol Med, 2003; 3: 127- 138.
8. Kefei KAM, Susan TN, Seth RS et al, Atopic dermatitis. Papulosquamous and eczematous dermatoses in Dermatology, Ed Bolognia JL, Jorizzo JL, Rapini RP, Mosby, 2006; pp199.
9. Yildiz L, Baris S, Senturk N et al, Overexpression of Bcl-2 in lymphocytes of psoriatic skin, J Europ Acad Dermatol Venereol, 2003; 17: 538.
10. Leung DY, Boguniewicz M, Howell MD et al, New insights into atopic dermatitis, J Clin Invest, 2004; 113: 651- 657.
11. Leung DY, Hanifin JM, Charlesworth EN, et al, Disease management of atopic dermatitis: a practice parameter, Ann Allergy Asthma Immunol, 1997; 79: 197- 211.
12. Knoell KA, Greer KE, Atopic dermatitis, Pediatr Rev, 1999; 20: 46- 52.
13. Jung T, New treatments for atopic dermatitis, Clin Exp All, 2002; 32: 347- 354.
14. Stoppolino G, Prisco F, Santinelli R, et al, Potential hazards of topical steroid therapy, Am J Dis Child, 1983; 137: 1130- 1131.
15. Ruzicka T, Bieber T, Sch?pf E et al, A shortterm trial of tacrolimus ointment for atopic dermatitis, European Tacrolimus Multicenter Atopic Dermatitis Study Group, N Engl J Med, 1997; 337: 816- 821.
16. Luger T, van Leent EJM, Graeber M, et al, SDZ ASM 981: an emerging safe and effective treatment for atopic dermatitis, Br J Dermatol, 2001; 144: 788- 794.
17. Grassberger M, Steinhoff M, Schneider D, et al, Pimecrolimus-antiinflammatory drug targeting the skin, Exp Dermatol, 2004; 13(12): 721- 730.
18. Kimberley FC, Screaton GR. Following a TRAIL: Update on a ligand and its five receptors, Cell Res, 2004; 14: 359- 372.
19. Mirandola P, Ponti C, Gobbi G, et al, Activated human NK and CD8+T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity, Blood, 2004; 104: 2418- 2424.
20. Mariani SM, Krammer PH, Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells, Eur J Immunol, 1998; 28: 1492- 1498.
21. Kemp TJ, Moore JM, Griffith TS, Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation, J Immunol, 2004; 173: 892- 899.
22. Tecchio C, Huber V, Scapini P, et al, IFN alpha-stimulated eosinophils and CD4 + T cells release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on HIV infected cells, Blood, 2007; 126: 2830- 2838.
23. Fanger NA, Maliszewski CR, Schooley K, et al, Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), J Exp Med, 1999; 190: 1155- 1164.
24. Warnnissorn P, Nakao A, Suto H, et al, Tumor necrosis factor-related apoptosis-inducing ligand expression in atopic dermatitis, Br J Dermatol, 2003; 148: 829- 831.
© 2009 Egyptian Dermatology Online Journal |















