|
|
Abstract
Malignant eccrine spiradenoma (MES; spiradenocarcinoma) is an extremely
rare tumor, which almost always arises from a preexisting eccrine spiradenoma
(a benign sweat gland tumor that commonly affects young adults) [1].
We describe a case of malignant eccrine spiradenoma that arose in the right
lateral thigh of a 62-year-old woman. The mass, which was present for approximately
35 years, suddenly enlarged with ulceration of the overlying skin. Microscopically,
the tumor showed the typical features of an eccrine spiradenoma with areas
of adenocarcinoma, squamous differentiation and sarcoma. The tumour qualified
for the designation carcinosarcoma arising in eccrine spiradenoma.
Introduction
Eccrine spiradenoma is a benign sweat gland tumor arising from the intradermal
straight part of the duct of eccrine sweat glands[1].
The presentation is often that of a single nodule that may or may not be
tender. Although benign forms are well recognized, malignant eccrine spiradenoma
(MES) is extremely rare and is usually a carcinoma [2,3,4].
Rare cases can have a carcinosarcomatous pattern [5,6,7].
In this report, we present an additional case of MES with carcinosarcomatous
pattern and review the literature.
Case Report
A 62-year-old woman presented with a 3 month history of a progressively
increasing painless nodule in her right lateral aspect of thigh, which had
assumed the form of a large nodular lesion with an ulcerated surface. A
small pea sized nodule had been present since approximately 30 years and
for the past 4 months, the lesion had abruptly started to enlarge, with
recent ulceration and bleeding. There were no palpable inguinal lymph nodes.
An incisional biopsy was performed which showed predominantly anaplastic
spindle cells with frequent mitoses and areas of haemorrhage and necrosis.
The lesion was reticulin rich with few reticulin poor clusters. It was reported
as malignant neoplasm NOS, and excision was advised.
Subsequently the excision specimen was received. The specimen with overlying
skin ellipse, measuring 6.2x 4.5x 3.7 cm, was received in the surgical pathology
laboratory. The skin surface showed a nodulo- ulcerative, exophytic, polypoidal
growth. The overlying skin was stretched out and showed an ulcer 1.5 cm
diameter, covered with exudate. On serial sectioning, a partially encapsulated,
multiloculated, cystic and solid, tan-yellow mass measuring 3x3.5x 3.5 cm
was noted. The lesion grossly involved the deep dermis and the subcutaneous
tissue. However, the margins of resection including the deep resection plane
were not involved.
On microscopic examination, the dermis and subcutaneous tissue showed
a tumor arranged in ragged sheets, nests, cords, and solid masses with central
necrosis along with occasional irregular glandular structures (Figure
1). Prominent areas with pleomorphic spindle cells in palisades were
observed along with few small foci with squamous differentiation. Areas
of tumor necrosis, cystic degeneration, and old hemorrhage were present.
The tumor cells had pleomorphic, vesicular nuclei with variably prominent
nucleoli and moderate amount of cytoplasm. The mitotic activity was high
(both in carcinomatous and sarcomatous areas), however, no lymphovascular
invasion was recognized. The overlying epidermis was ulcerated, but did
not show any dysplasia. Adjoining to this high-grade carcino-sarcoma, benign
eccrine spiradenoma, was seen as sharply demarcated lobules composed of
2 cell populations (peripheral basaloid cells and central pale cells) arranged
in nests and inter- anastomosing trabeculae with scattered glandular lumina
and focal cystic changes (Figure 2). The spindle cell areas were
reticulin rich in contrast to the carcinomatous areas, which were reticulin
poor (Figure 3). Immunohistochemically the sarcomatous areas were
positive for Vimentin and negative for smooth muscle Actin (SMA) and Desmin.
The carcinomatous areas were negative for Vimentin.
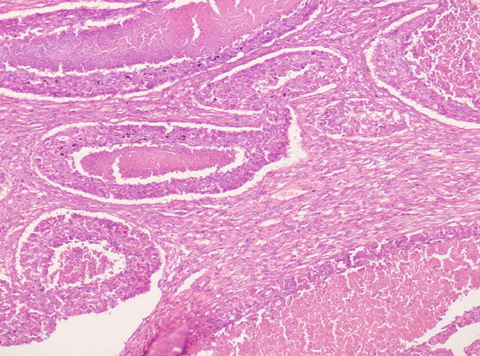 | Fig 1:
Photomicrograph showing carcinoma with central necrosis and
adjacent area showing sarcomatous change (H&E x 100). |
|
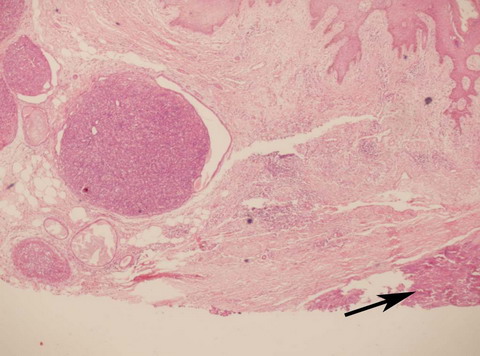 | Fig
2:
Photmicroograph showing sharply demarcated lobules of benign
Eccrine Spiradenoma with a malignant focus shown by pointer (H&E
x 40). |
|
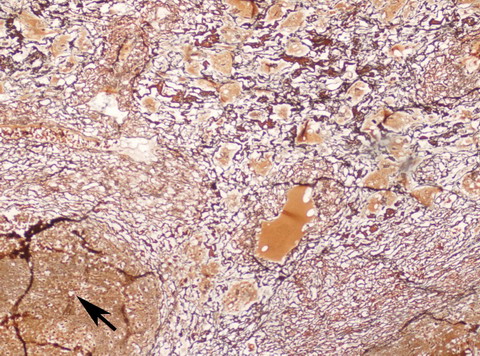 | Fig
3:
Photomicrograph showing a focus of carcinoma shown by pointer
surrounded by reticulin rich sarcomatous areas with prominent
vascularity (Reticulin x 100). |
|
Metastatic workup (bone scan, computed tomographic scans of the chest,
abdomen, and pelvis) was negative.
Discussion
The motive behind presenting this case was to highlight that malignant
transformation in eccrine spiradenoma can have the appearance of carcinosarcoma,
with ulceration of the overlying epidermis. The malignant eccrine spiradenoma
almost always arises from a pre- existing benign eccrine spiradenoma after
a variable latent period, which may be as long as 75 years.[8]
It generally begets medical attention when a pre-existing undiagnosed lesion
rapidly enlarges, changes color, ulcerates, or becomes painful and tender.
This malignant transformation is, fortunately, extremely rare. According
to the estimates of Marenda and Otto[9],
malignant sweat gland tumors account for only 0.005% of all skin tumors.
Clinically, MES presents at an average age of 59 years (range, 21- 92
years) and shows no sex predilection. The tumor usually presents as a solitary
firm round dermal nodule on any part of the body but most frequently on
the face, scalp, trunk and proximal parts of limbs (92% of reported cases)[2].
Infrequently multiple lesions are present which may become confluent or
remain discrete. The overlying epidermis may show no change in color or
may be pinkish and rarely ulcerated, as was seen in the present case. The
average size of MES at presentation is 3.9 cm (range, 0.5-15 cm) [2].
Diagnosis of MES is based on histopathologic examination and requires finding
a focus of benign spiradenoma within or adjacent to the malignant tumor.[2]
Histologically, proliferation of cells with hyperchromatic nuclei, increased
mitoses, loss of Periodic-Acid-Schiff positive basement membrane, and invasion
of the surrounding tissues characterize malignant transformation in eccrine
spiradenoma. MES frequently shows focal squamous differentiation, which
may be florid in rare instances.[8] The
malignant transformation is usually into a carcinoma; however, carcinosarcomatous
transformation has also been reported, as seen in our case. The mesenchymal
elements are often nonspecific spindle cells, but rhabdomyosarcoma, osteosarcoma,
leiomyosarcoma, and chondrosarcoma have been described in association with
carcinoma arising in eccrine spiradenoma.[10,7,5,8,3]
In the absence of a benign focus, the tumor with carcino-sarcoma pattern
can be confused microscopically with other malignancies such as squamous
cell carcinoma, synovial sarcoma, epithelioid sarcoma and metastatic carcinomas.
MES may also be highly vascular and increased vascularity may also lead
to diagnostic confusion with a vascular neoplasm.[11]
Immunohistochemically, these tumors exhibit variable expression of cytokeratins,
carcinoembryonic antigen, epithelial membrane antigen, and S100 protein.[11,5,8,3]
Overexpression of p53 protein in MES has been associated with malignant
transformation.[8] Recently these tumors
have been shown to demonstrate estrogen receptor positivity, and hormonal
receptor status should be evaluated for potential therapeutic options.[11]
Malignant eccrine spiradenoma metastasizes to regional lymph nodes, lungs,
brain, and liver (in a descending order of frequency) [2].
While distant metastases of MES are uncommon, they generally portend an
ominous prognosis. Appropriate therapy of malignant eccrine spiradenoma
consists of a wide local excision with resection of clinically suspicious
lymph nodes. Irradiation of the resection site can be useful in preventing
local recurrence. The role of chemotherapy is not yet clearly defined. Symptomatic
improvement and shrinkage of the tumor with tamoxifen therapy in a patient
with estrogen receptor-positive eccrine adenocarcinoma has also been reported
[2]. However, the roles of hormonal therapy
and other modalities, such as localized postoperative radiation therapy,
prophylactic lymph node dissection and chemotherapy still remain to be determined.
Close follow-up of these patients for early detection of recurrence and
metastases is recommended.
References
1. Agarwal S, Khanna R, Arya NC, Khanna AK. Malignant eccrine
spiradenoma. An unusual presentation. Indian J Dermatol Venereol Leprol.
2002; 68: 290- 291.
2. Mirza I, Kloss R, Sieber SC. Malignant eccrine spiradenoma.
Arch Pathol Lab Med 2002. 126 (5): 591- 594.
3. Fern?ndez-Ace?ero MJ, Manzarbeitia F, Mestre de Juan
MJ. et al. Malignant spiradenoma: report of two cases and literature review.
J Am Acad Dermatol 2001; 44: 395- 398.
4. Granter SR, Seeger K, Calonje E. et al. Malignant eccrine
spiradenoma (spiradenocarcinoma): a clinicopathologic study of 12 cases.
Am J Dermatopathol 2000; 22: 97- 103.
5. McCluggage WG, Fon LJ, O'Rourke D. et al. Malignant eccrine
spiradenoma with carcinomatous and sarcomatous elements. J Clin Pathol 1997;
50: 871- 873.
6. Saboorian MH, Kenny M, Ashfaq R. et al. Carcinosarcoma
arising in eccrine spiradenoma of the breast. Arch Pathol Lab Med 1996;
120: 501- 504.
7. Itoh T, Yamamoto N, Tokunaga Y. Malignant eccrine spiradenoma
with smooth muscle differentiation: histological and immunohistochemical
study. Pathol Int 1996; 46: 887- 893.
8. Ishikawa M, Nakanishi Y, Yamazaki N. et al. Malignant
eccrine spiradenoma: a case report and review of the literature. Dermatol
Surg 2001; 27: 67- 70.
9. Marenda SA, Otto RA. Adnexal carcinomas of the skin.
Otolaryngol Clin North Am 1993; 26: 87- 116.
10. McKee PH, Fletcher CDM, Stavrinos P. et al. Carcinosarcoma
arising in eccrine spiradenoma: a clinicopathological and immunohistochemical
study of two cases. Am J Dermatopathol 1990; 12: 335- 343.
11. Malhotra R, Kumar W, Wilbum S. et al. Malignant eccrine
spiradenomas with hemangiomatous elements: DNA ploidy, light microscopy,
and immuno-histochemical studies of two cases. J Cutan Pathol 1993; 20:
555.© 2009 Egyptian Dermatology Online
Journal
|



