|
|
Abstract:
Complement has both beneficial and deleterious roles in the pathogenesis
of systemic lupus erythematosus (SLE). On the one hand, patients with SLE
present with decreased complement levels and with complement deposition
in inflamed tissues, suggestive of a harmful role of complement in the effector
phase of the disease. On the other hand, homozygous deficiency of any of
the classical pathway proteins is strongly associated with the development
of SLE.
The homozygous C2 deficiency is the most common deficits as a fraction
of complement associated with lupus. We report two children with chronic
lupus erythematosus and deficiency of C2. The mechanisms behind this association
and the clinical and immunological particularity are discussed.
Introduction:
The complement plays important role in the defence of the host against
infection and the elimination of immune complexes. Therefore the exploration
of the components of the complement system is indicated in cases of repeated
infections, auto-immune diseases particularly systemic lupus erythematosus
(SLE) or in the glomerulopathies.
The association between complement deficiencies and SLE could be explained
by several mechanisms, including impaired clearance of immune complexes
and impaired handling of apoptotic cells, aberrant tolerance induction or
changes in cytokine regulation.
We report two cases of chronic lupus erythematosus and C2 deficiency
in two children.
Case 1:
A 10-year old female was followed in the dermatology department for skin
lesions, appeared a year earlier, in the form of erythemato- papular eruption
affecting the nose and cheeks (Fig. 1), erythematosus finely scaly hands
and feet (Fig. 2a and 2b). There was a scaly patch of alopecia of the scalp,
the direct microscopic examination KOH preparations from hair and scales
ware negative for fungus.
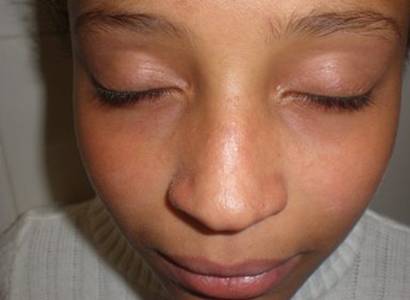 | Fig 1:
Erythemato- papular eruption affecting the nose and cheeks. |
|

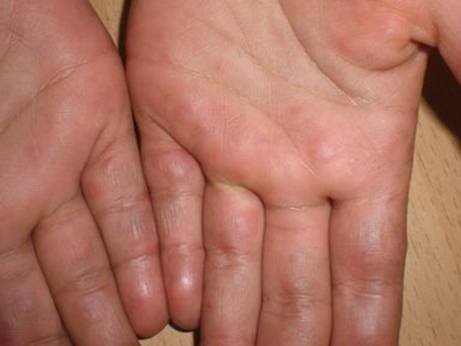 |
Fig 2a and Fig 2b: Erythematosus finely scaly hands closets. |
|
The histology showed: vacuolar alteration of the basal cell layer, thickening
of the basement membrane, hyperkeratosis, atrophy of the epidermis, incontinence
of pigment, and inflammatory lymphocytic cell infiltrate in a perivascular,
periappendiceal, and subepidermal location that was in favor of chronic
discoid lupus erythematosus (Fig 3), and direct immuno-fluorescence showed
micro granular IgG, IgM weak fluorescence and C3 at the dermo-epidermal
junction.
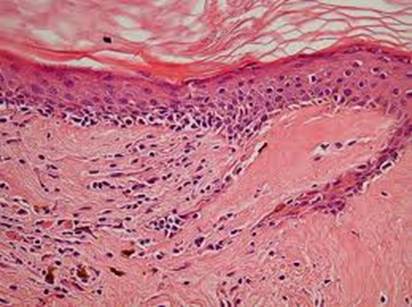 | Fig 1:
Histopathology showing chronic lupus erythematosus |
|
Laboratory studies showed weakly positive antinuclear antibody at 1 /
160, positive Anti-Ro (SS-A) antibodies. The anti-nDNA antibodies were absent.
Her serum showed homozygous C2 deficiency: Functional C2 activity < 10%
(normal 70-115%). C3 =1, 401 g / l (normal: 0,743-1.62), C4= 0,155 g / l
(normal: 0,162-0,530).
Her father has a functional C2 to 22%.
No renal or cerebral or pleuropericardial involvement were observed during
evolution.
The patient was treated with hydroxychloroquine (Plaquenil ®) at a dose
of 4 mg/kg/day and external photoprotection. The evolution was marked by
the disappearance of skin lesions.
Case 2:
A 9-year old female was followed for 2 years in the dermatology department,
for scaly erythemato- papular plates, affecting the nose and cheekbones.
Skin biopsy and direct immuno-fluorescence showed chronic lupus erythematosus.
Laboratory analysis showed weakly positive antinuclear antibody. The Anti-Ro
and anti-nDNA antibodies were absent. C3: 1, 070 g / l, C4: 0,176 g / l,
CH50 = 0% (normal: 75-138%), functional C2 at 0% and a homozygous C2 deficiency
type 1. A rigorous photoprotection was advocated associated with hydroxychloroquine.
Discussion:
Homozygous deficiency of each of the classical pathway complement components
(C1q, C1r, C1s, C4, C2) is associated with an increased susceptibility to
SLE [1,2].
The most common homozygous complement deficiency is that of C2, which
occurs in 1: 10,000 to 20,000 individuals. Heterozygotes are present in
1 to 2% of the normal Caucasian population. SLE occurs in 10 to 30% of homozygous
C2 null individuals [2,3,4].
Of patients with SLE, C2 deficiency accounts for approximately 1% [5].
Heterozygotes do not appear to be at an increased risk for SLE [5].
Inherited deficiencies of C1q and C4 are invariably associated with the
development of a severe, lupus-like disease early in life, while C2 deficiency
is only weakly associated with a milder form of SLE, an association which
has most likely been overestimated [2].
In contrast, whereas deficiency of C3 predisposes to recurrent pyogenic
infections and membrano-proliferative glomerulonephritis, it is rarely associated
with SLE [2].
The association between complement deficiency and SLE appears even more
paradoxical: complement deficiency causes SLE, and yet SLE causes activation
and consumption of complement. These clinical observations suggest that
the early part of the classical pathway plays a key protective role against
the development of SLE. A recently described link between the complement
system and apoptosis may explain these apparently paradoxical findings [6].
The reasons for the development of SLE in classical pathway deficiencies
have been subject to extensive study [2,5,6,7,8].
All findings are compatible with the hypothesis that complement deficiency
causes SLE by impairment of the physiological clearance of apoptotic cells
by macrophages. These uncleared apoptotic bodies in turn may provide the
source of the autoantigens that drive the autoimmune response of SLE. A
reduced ability of macrophages to remove apoptotic cells at sites of inflammation
may promote the clearance of these cells by pro-immune antigen-presenting
cells, and if the necessary pro-inflammatory cytokine milieu is present,
this may drive dendritic cell maturation and initiate an autoimmune response
followed by tissue damage and complement activation.
The clinical findings observed in SLE patients with C2 deficiency include
early onset, skin lesions resembling discoid lupus, photosensitivity, mild
renal, cerebral and pleuropericardial involvement and repeated bacterial
or viral infections. Antinuclear antibody titres are usually low and anti-nDNA
antibodies are absent. Anti-Ro (SS-A) antibodies, however, have been found
in a large proportion (up to 73%) of patients with SLE and C2 deficiency
[5,9,10].
In SLE without a complement component deficiency, anti-Ro antibodies
are observed in only 20-30% [9,11]
and in Sjogren's syndrome in 40-70% [9,12].
A high incidence (62%) of anti-Ro antibodies have also been described in
patients with subacute cutaneous lupus erythematosus [13].
Inherited deficiency of C2 was suspected when the C2 protein was low
(less than 2 SD below the mean) or low normal (less than 1 SD below the
mean), particularly if other components of the classical pathway were within
the normal range [14]. Functional C2 activity
was then measured in those individuals with this apparently isolated deficiency
of C2 or a low normal value. If the functional C2 level was less than 2
SD below the mean, multiple samples stored at intervals during routine clinical
follow-up were analyzed retrospectively by both C2 protein and functional
assays to exclude acquired changes in C2 levels [14].
The C2 gene is located in the middle of the major histocompatibility
complex (MHC) class III region together with the genes for C4 and factor
B. Two principal variants of C2 deficiency have been distinguished: type
I is characterized by the absence of detectable C2 synthesis, while type
II is caused by a selective block of C2 secretion [15].
Conclusion:
C2 deficiency is associated with many diseases e.g. vasculitis, glomerulonephritis,
dermatomyositis, but association with discoid or systemic lupus erythematosus
is the most common. Lupus associated with C2 deficiency has clinical and
immunological particularity.
Homozygous deficiency in one or several fractions of complement is usually
suspected when CH50 was low.
References
1. Walport MJ, Morgan BP: Complement deficiency and disease.
Immunol Today 1991; 12: 301- 306.
2. Pickering MC, Botto M, Taylor PR, et al.: Systemic lupus
erythematosus, complement deficiency, and apoptosis. Adv Immunol 2000; 76:
227- 324.
3. O'Neil KM: Complement deficiency. Clin Rev Allergy Immunol
2000; 19: 83- 108.
4. Sullivan KE: Complement deficiency and autoimmunity.
Curr Opin Pediatr 1998; 10: 600-606.
5. Maria-Louise Barilla-LaBarca, MD, John P. Atkinson, MD.
Rheumatic syndromes associated with complement deficiency. Current Opinion
in Rheumatology 2003; 15: 55- 60.
6. Botto M. Links between complement deficiency and apoptosis.
Arthritis Res 2001; 3: 207-210.
7. Sturfelt G, Bengtsson A, Klint C, Nived O, Sjoholm A,
Truedsson L. Novel roles of complement in systemic lupus erythematosus-hypothesis
for a pathogenetic vicious circle. J Rheumatol. 2000; 27: 661- 663.
8. Taylor PR, Carugati A, Fadok VA et al. A hierarchical
role for classical pathway complement protein in the clearance of apoptotic
cells in vivo. J Exp Med 2000; 192: 359- 366.
9. Reichlin M. Clinical and immunological significance of
antibodies to Ro and La in systemic lupus erythematosus. Arthritis Rheum
1982; 25: 767.
10. Provost T.T., Arnett F.C. & Reichlin M. Homozygous
C2 deficiency, lupus erythematosus, and anti-Ro (SSA) antibodies. Arthritis
Rheum 1983; 26 :1279.
11. BellD.A. and Maddison P.J. Serologic subsets in systemic
lupus erythematosus. Arthritis Rheum 1980; 23: 1268.
12. Alexander E.L, Arnett F.C, Provost T.T. Sjogren's syndrome:
association of anti-Ro (SS-A) antibodies with vasculitis, hematologic abnormalities,
and serologic hyperreactivity. Ann. Intern. Med 1983; 98: 155.
13. Sontheimer R.D., Maddison P.J., Reichlin M., et al.
Serologic and HLA associations in subacute cutaneous lupus erythematosus,
a clinical subset of lupus erythematosus. Ann. Intern. Med 1982; 97: 664.
14. Glass D, Raum D, Gibson D, Stillman J.S, and Schur
P. H. J. Inherited Deficiency of the Second Component of Complement. Clin
Invest.1976; 58: 853- 861.
15. Yu CY. Molecular genetics of the human MHC complement
gene cluster. Exp Clin Immunogenet. 1998; 15: 213- 230.
© 2010 Egyptian Dermatology Online Journal
|




