|
|
Abstract
A 51-year-old female patient presented with typical lesions of nodular vasculitis, which was confirmed by histopathology, and was proved to be of non-tubercular etiology by the absence of mycobacterial DNA in tissue PCR. The patient was initially treated with saturated solution of potassium iodide which led to partial improvement, but it had to be discontinued due to gastric irritation. The patient was then put on dapsone, starting with 100 mg per day, following which good response was seen. Introduction
Nodular vasculitis (NV), also known as 'erythema induratum', is characterized by a chronic relapsing lobular panniculitis with septal vasculitis, and is believed to be due to a hypersensitivity reaction to antigenic triggers such as bacterial infections [1]. Erythema induratum due to tuberculosis is termed erythema induratum of Bazin, whereas that due to a non-tubecular etiology is termed erythema induratum of Whitfield [2]. Treatment, with the limited available options, may, at times, be difficult and frustrating. Here we describe a patient of NV of non-tubercular etiology who responded well to dapsone.
Case Report A 51-years-old female patient presented with multiple, painful, reddish nodules of four months duration in the posterior aspects of both legs. There was no history of preceding infection or exacerbation of the skin lesions on exposure to cold. The general physical and systemic examination findings were normal; except for obesity (body mass index was 31.2). On cutaneous examination, multiple, erythematous and violaceous nodules and plaques measuring 1 cm to 7 cm were seen on the posterior aspect of both legs along with post-inflammatory hyper-pigmentation and atrophic changes of healed lesions (Figure 1). There was no evidence of ulceration, livedo pattern at the periphery, erythrocyanosis, or perifollicular erythema.
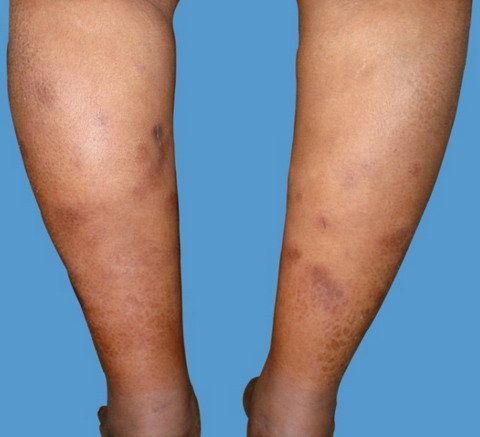 | Fig 1:
Multiple, erythematous and violaceous nodules and plaques on the
posterior aspect of both legs. |
|
Laboratory investigations revealed a raised erythrocyte sedimentation rate (46 mm/hr). An elaborate work up for foci of infections yielded no result. The tuberculin test was negative. In histopathology, predominant changes were seen in the subcutis - septal infiltration by lymphocytes and neutrophils along with vasculitis of the septal blood vessels (Figure 2a), and the presence of similar infiltrates within the fat lobules in between the individual fat cells (Figure 2b). There was no granuloma in the lesion. The histopathology was diagnostic of nodular vasculitis. Real time PCR of a skin biopsy specimen for Mycobacterium genus and Mycobacterium tuberculosis complex (M. tuberculosis, M. bovis, M. microti, M. africanum) was negative, thereby confirming a non-tubercular etiology.
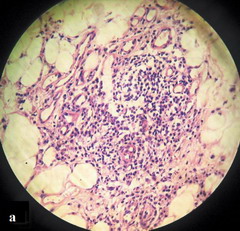 | Fig
2a: Septal inflammation with vasculitis of the septal blood
vessels (H&E X 400). |
|
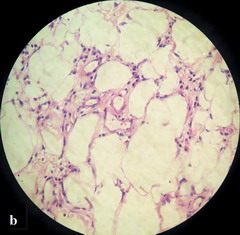 | Fig
2b: Inflammatory cell infiltrates within the fat
lobules in between the individual fat cells (H&E X 400). |
|
Systemic corticosteroids were not considered for the treatment because the patient was diabetic and hypertensive. She was started on saturated solution of potassium iodide to which she responded initially, but, it had to be discontinued due to gastric irritation, resulting in exacerbation of the lesions. Dapsone was then started at a dose of 100 mg per day. The patient responded well to dapsone, and after 3 months of the therapy the skin lesions as well as pain and tenderness had completely subsided. The dose of dapsone was then tapered, and the patient was lesion-free when followed up 6 weeks later on a tapering dose of dapsone 50 mg per day (Figure 3). After 2 months of further follow up on 50 mg of dapsone per day, the patient was free of lesions.
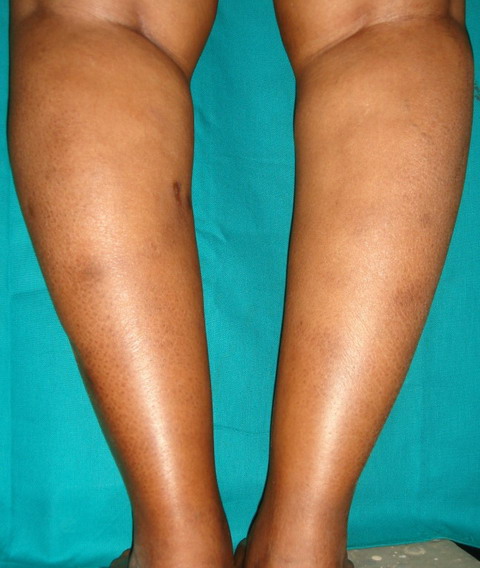 | Fig
3: Healed skin lesions following dapsone therapy (at 8 months). |
|
Discussion
The term erythema induratum was coined by Bazin in 1861 to describe a cutaneous reaction pattern which was later believed to be associated with tuberculosis [1,3]. Another clinically and histopathologically similar condition not associated with tuberculosis was described by Whitfield in the early twentieth century [1]. Recently, these two disorders have been considered by American authors under the same umbrella term "nodular vasculitis" [2]. NV is regarded as a form of cutaneous vasculitis [1,4], wherein vasculitis of the subcutaneous vessels (arteries and veins) results in ischemia of the subcutaneous fat, thereby producing clinical suppuration [1]. The pathogenesis of NV may involve a delayed hypersensitivity reaction or an Arthus type reaction [5,7], with perpetuation of the disease even after the infection has cleared due to the host's response [2,5,6]. Most non-tuberculous cases are idiopathic [2], but anecdotal reports of NV associated with underlying streptococcal, hepatitis C virus infection [5,8] and drugs like propylthiouracil have been described [1,2]. Rheumatoid arthritis may be associated with a lobular panniculitis with vasculitis similar to NV [2]. NV has also been reported to occur with ulcerative colitis [9] and colonic adenocarcinoma [10]. Erythema induratum occurring in women under the age of 25 is believed nearly always to be tuberculous in origin, and that over 30 is nearly always not [2,11]. Individuals with NV tend to have no systemic involvement, but the condition tends to occur in cold, winter months and has a prolonged recurrent course over many years, with new crops of lesions occurring at irregular intervals [2]. The diagnosis of NV is always confirmed by histopathology which typically shows lobular or septo-lobular panniculitis with septal vasculitis, granulomatous inflammation, focal necrosis and septal fibrosis [5,12]. There is no consistent histological finding to differentiate the tubercular type from the non-tubercular type. However, necrosis and the presence of a tuberculoid granuloma in the deep dermis, often around the eccrine glands, may favor a tubercular etiology [5]. PCR amplification for the detection of mycobacterial DNA in the skin biopsy specimens is important in establishing a tubercular etiology. Baselga et al showed that PCR amplification were positive for Mycobacterium tuberculosis complex DNA in 77% of the skin biopsy specimens in patients with lobular granulomatous panniculitis (NV) [6]. The treatment of NV of non-tubercular etiology includes compression hosiery, bed rest, non-steroidal anti-inflammatory drugs, and treatment of any identifiable precipitating factors [1]. Systemic steroids and potassium iodide may also be considered [1]. Recently, mycophenolate mofetil has been reported to be effective in nodular vasculitis [13]. Drugs which are effective in cutaneous small vessel venulitis (CSVV) may also be tried for the treatment of NV [1]. Dapsone, in a dose of 100-200 mg/day, is primarily an anti-inflammatory medication with a predominantly anti-neutrophilic effect that is frequently used in various vasculitic diseases and neutrophilic dermatoses [14], and usually considered a first line drug for the treatment of CSVV involving predominantly the skin [15]. The main mechanism of action of dapsone is inhibition of myeloperoxidase-H2O2-halide-mediated cytotoxic system of the neutrophils thereby reducing the production of free oxygen radicals, which damage tissues in inflammation [16]. Dapsone also inhibits neutrophil chemotaxis and stabilizes the lysozymes of neutrophils [16]. Another possibility for the beneficial effect of dapsone on neutrophil-rich dermatoses may be its ability to inhibit interleukin 1 stimulated neutrophil adhesion to endothelial cells, neutrophil adherence to antibodies deposited in skin, integrin-mediated neutrophil adherence functions and chemoattractant-induced signal transduction [17]. The decision to treat our patient with dapsone was based on the histopathological finding of neutrophilic infiltrate in the skin lesions. Our case highlights the fact that dapsone could be effective in treating non-tubercular NV, and is a useful alternative especially when systemic corticosteroids are contraindicated. References
1. Barham KL, Jorizzo JL, Grattan B, Cox NH. Vasculitis and neutrophilic vascular reactions. In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook's Textbook of Dermatology, 7th edn., Oxford: Blackwell Science 2004: 49.1- 49.46
2. Camilleri MJ, Su WPD. Panniculitis. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick's Dermatology in General Medicine, 6th edn., NewYork: McGraw-Hill 2003: 1047- 1063.
3. Lee YS, Lee SW, Lee JR, Lee SC. Erythema induratum with pulmonary tuberculosis: histopathologic features resembling true vasculitis. Int J Dermatol 2001; 40: 193- 196.
4. Gibson LE, Su WPD. Cutaneous vasculitis. Rheum Dis Clin North Am 1995; 21: 1097-1113.
5. Weedon D. Skin Pathology, 2nd edn., London: Churchill- Livingstone 2002: 521-541.
6. Baselga E, Margall N, Barnadas MA, Coll P, de Moragas JM. Detection of Mycobacterium tuberculosis DNA in lobular granulomatous panniculitis (erythema induratum-nodular vasculitis). Arch Dermatol 1997; 133: 457- 462.
7. Cho KH, Lee DY, Kim CW. Erythema induratum of Bazin. Int J Dermatol 1996; 35: 802- 808.
8. Ural I, Erel A, Ozenirler S, Tekin NS, Gurert MA. Nodular vasculitis associated with chronic hepatitis C. J Eur Acad Dermatol Venereol 2002; 16: 298- 299.
9. Pozdnyakova O, Garg A, Mahalingam M. Nodular vasculitis - a novel cutaneous manifestation of autoimmune colitis. J Cutan Pathol 2008; 35: 315- 319.
10. Khachemoune A, Longo MI, Phillips TJ. Nodular vasculitis as a paraneoplastic presentation? Int J Dermatol 2003; 42: 639- 642.
11. Bettley FR. Erythema induratum (Whitfield). Proc R Soc Med 1950; 43: 391-2.
12. McNutt NS, Moreno A, Contreras F. Inflammatory diseases of the subcutaneous fat. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, eds. Lever's Histopathology of the Skin, 9th edn., Philadelphia: Lippincott Williams & Wilkins 2005: 519- 549.
13. Taverna JA, Radfar A, Pentland A, Poggioli G, Demierre MF. Case reports: nodular vasculitis responsive to mycophenolate mofetil. J Drugs Dermatol 2006; 5: 992- 993.
14. Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol 2006; 45: 3-13.
15. Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol 2008; 9: 71- 92.
16. Wolf R, Matz H, Orion E, Tuzun B, Tuzun Y. Dapsone. Dermatol Online J 2002; 8(1): 2.
17. Katz SI. Sulfones. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick's Dermatology in General Medicine, 6th edn., NewYork: McGraw-Hill 2003: 2388- 2392.© 2010 Egyptian Dermatology Online Journal |




