|
|
Abstract
Background and Objectives: Onychomycosis, a fungal infection of
nails occurs worldwide with dermatophytes as the most common causal agents,
although yeasts and moulds are also involved. The diagnosis usually involves
direct microscopy and culture to prove actual existence of onychomycosis.
This present study aims to provide knowledge of the epidemiology and mycological
characteristics of onychomycosis involving toenails among rural farmers
in a southwestern part of Nigeria.
Methods: Direct microscopic examination using 20% potassium hydroxide
(KOH) and mycological culture of 631 sub-ungual scrapings and toenail clippings
from farmers suspected to have onychomycosis were prepared to identify the
causative agents.
Results: Trichophyton rubrum occurred as the predominant species
of the total dermatophytes isolated (63.8%). The highest prevalence of individual
etiologic agent was Trichophyton rubrum (16.9%), followed by Candida albicans
(14.6%). One hundred and six non-dermatophyte moulds, of which Aspergillus
niger and Fusarium moniliforme were the most frequent species (13.4% and
7.3% respectively) were also recovered from the specimens. Distal and lateral
sub-ungual onychomycosis (DLSO) was the most prevalent clinical form occurring
in 166 (63.6%) of the positive cultures followed by white superficial onychomycosis
(WSO) in 51 (19.5%).
Conclusion: The study revealed that dermatophytes and Candida
species are involved in the pathogenesis of toenail infections among rural
farmers in southwestern Nigeria. The occurrence of non-dermatophytic moulds
should also not be ignored.
Introduction
Onychomycosis is a general term used for any fungal infection of the
nail plate. This contrasts with the term tinea unguium which is specifically
used for infection of the nails caused by dermatophytes. Zaias [1]
divided onychomycosis into four clinical types; distal sub-ungual onychomycosis,
which is the most common type of onychomycosis caused by dermatophytes,
Candida species and miscellaneous mould and yeasts; proximal sub-ungual
onychomycosis caused exclusively by dermatophytes and is the least common;
white superficial onychomycosis; and candidal onychomycosis caused mainly
by Candida albicans and non-albican species such as C. parapilosis, C krusei
and C. gullermondii [2]. Onychomycosis accounts
for 50% of all nail disorders [3,4]
with those affecting the toenails being 25 times more than that of the fingernails
[5].They are mainly caused by dermatophytes.
The dermatophytes commonly associated with onychomycosis include Trichophyton
rubrum and T. mentagrophytes with the former occurring in up to 90% of cases
[6,7]. Onychomycosis
are caused, to a lesser extent, by moulds and yeasts. The moulds most frequently
isolated from diseased nails are Aspergillus spp., Fusarium spp, Scopulariopsis
brevicaulis, Scytalidium dimidiatum, S. hyalinum, Onychocola canadensis
[8,9,10,11,12,13].
Reports in the literature revealed geographical differences in the epidemiology
of onychomycosis. The clinical patterns of onychomycosis are changing because
of the involvement of non-dermatophytic moulds. In Nigeria, most of the
research work done on the superficial mycoses has been on the skin. There
is paucity in the literature on reports on onychomycosis in Nigeria. The
reports of Gugnani et al., [14]
and Greer [15] were the only reports in
the literature known to the authors in Nigeria. Therefore this study was
undertaken to investigate the prevalence of onychomycosis among rural farmers,
who, through the authors interaction were observed to have conspicuous black
pigmentation of toenails accompanied, in some cases, by painful inflammation
and decay of underlying tissues, resulting in fall-off of the nails. This
to the authors' knowledge is the first report in southwestern Nigeria documenting
fungi from toenails of farmers suspected to have onychomycosis.
Materials And Methods
Specimen collection:
The study was carried out in three villages located in Akinyele Local
Government in lbadan, southwestern Nigeria between May 2002 and March 2006.
Farmers, all of who were males between the ages of 25-67 years with discoloured
toenails and sometimes with painful inflammation and decayed underlying
tissues were included in this study. A total of 631 sub-ungual scrapings
and toenail clippings were collected in separate universal plastic bottles
(Sayag, France) after cleaning the affected areas with 95% ethanol and transported
to the laboratory immediately after collection.
Microscopy, culture and strain identification:
Direct microscopic examination was carried out on the specimens by dissolving
a portion of each sample in 20% potassium hydroxide (KOH) for 60 minutes
to be examined under low and high power microscope for the presence of fungal
elements such as hyphae, pseudo-hyphae, budding cells, spores or blastoconidia.
Culture of all specimens irrespective of direct microscopy results was carried
out on Dermasel agar (Oxoid), Sabouraud Dextrose Agar containing cycloheximide
(Oxoid) and Dermatophyte Test medium (DTM) [16].
The culture media were incubated at room temperature (28+ 20C) and examined
daily for two weeks. The change of colour from yellow to red on DTM indicated
the growth of a dermatophyte.
Macroscopic and microscopic examinations of fungal colonies were carried
out as follows. Wet mounts of moulds was carried out with Lactophenol cotton
blue. The moulds were identified further according to the taxonomic schemes
of Klich and Pitt [17] for Aspergillus
spp, Nelson et al. [18] for Fusarium
spp. All Candida albicans isolates were examined for germ-tube production
in human serum and for chlamydospores formation in corn-meal agar - Tween
80. Other yeasts species other than C. albicans were identified using their
sugar fermentation and assimilation profiles. The morphology of the dermatophytes
and other moulds as described by Al- Doory [16]
were employed for their identification.
Results
Out of the 631 sub-ungual scrapings/nail clipping examined, 261 samples
were positive for fungal growth. Mycological analysis showed the presence
of two main genera of filamentous fungi, Aspergillus and Fusarium. The genus
Fusarium was represented by three species, Fusarium oxysporum, F. solani
and F. moniliforme. Four species of Aspergillus were identified which include
Aspergillus niger, A. nidulans, A. terreus and A. fumigatus with A. niger
being the predominant species. Other genera of fungi isolated were Cladosporium,
Mucor and Penicillium. Candida albicans, C. tropicalis and Candida spp were
the yeasts encountered while dermatophytes were represented by Trichophyton
rubrum, T. mentagrophytes and Microsporum gypseum. T. rubrum occurred as
the predominant (63.8%) of total dermatophytes isolated. Mixed cultures
were obtained in 13 specimens. Fifteen fungal species remained unidentified.
As shown in Table 1, the highest incidence of fungi isolated was the moulds
(40.6%) followed by dermatophytes (26.4%) and then yeasts (22.2%). However,
the highest prevalence of individual etiologic agent was T. rubrum with
a total of 44 isolates (16.9%), followed by Candida albicans (14.6%), Aspergillus
niger (13.4%), Fusarium moniliforme (7.3%) and T. mentagrophytes (6.5%).
|
Organism (%)* |
No. of positive specimens |
Yeasts
Candida albicans (65.5)
C. tropicalis (13.8)
Candida spp. (20.7) |
38
8
12 |
Moulds
Aspergillus niger (33.0)
A. nidulans (6.6)
A. terreus (9.4)
A. fumigates (5.7)
Fusarium oxysporum (10.4)
F. solani (3.8)
F. moniliforme (17.9)
Penicillium chrysogenum (3.8)
Penicillium spp. (2.8)
Cladosporium spp. (1.9)
Mucor spp. (4.7) |
35
7
10
6
11
4
19
4
3
2
5 |
Dermatophytes
Trichophyton rubrum (63.8)
T. mentagrophytes (24.6)
Microsporum gypseum (11.6) |
44
17
8 |
|
Mixed cultures |
13 |
|
Unidentified |
15 |
* % incidence of each isolate was calculated based on the total no. of
isolates in each group.
Table 1: Incidence of fungi isolated from toe nails of farmers
with onychomycosis
Table 2 shows the age groups of farmers studied. Onychomycosis affected
all age groups with the highest frequency recorded for ages between 40-49
years. The ages of 21 farmers could not be ascertained.
| Age
group (years) |
Number with onychomycosis
(%) |
25-29
30-39
40-49
50-59
60-69
Age unknown |
29 (11.1)
58 (22.2)
73 (28.0)
42 (16.1)
38 (14.6)
21 (8.0) |
| Total |
261 (100) |
Table 2: Distribution of onychomycosis by age groups
Table 3 shows the clinical forms observed in farmers with onychomycosis.
Distal and lateral sub-ungual onychomycosis (DLSO) was the most prevalent
clinical form and the commonest organism isolated from DLSO was T. rubrum
followed by C albicans, A. niger and T. mentagrophytes var. interdigitale
in that order.
|
Clinical forms |
No. (%) |
Etiologic agent isolated |
| DLSO |
166
(63.6) |
T.
rubrum, T. mentagrophytes, C. albicans, A. niger |
| PSO
(without paronychia) |
28(10.7) |
T. rubrum, T. mentagrophytes. C. albicans, C.tropicalis |
| WSO
51 |
(19.5) |
T.
mentagrophytes, F. oxysporum |
|
TDO |
16 (6.1) |
T. rubrum, C. albicans |
Table 3: Clinical patterns of onychomycosis and etiologic agents
isolated
Discoloration of toe nails was seen mostly in subjects with DLSO.
The majority of the farmers with white superficial onychomycosis (WSO) had
T. mentagrophytes var. interdigitale and F. oxysporum as the causative agents
with the former predominating (67%).
Fig. 1 shows slide culture of Fusarium moniliforme isolated from toe
nail samples.
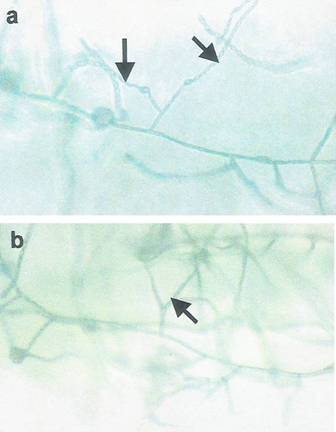
| Fig 1: Long and short chains of microconidia
of F. moniliforme on monophialides (a) as well
as the branched monophialides of the fungus (b)
on KCl medium. |
|
DLSO: distal and lateral sub-ungual onychomycosis; PSO: proximal sub-ungual
onychomycosis; WSO: white superficial onychomycosis; TDO: total dystrophic
onychomycosis.
Discussion
The results of this study showed that fungi of different genera were
prevalent in the toenails of farmers investigated. Results obtained with
direct microcopy did not correlate with the results obtained during culture
in some cases involving moulds. This in not surprising because reports have
shown that KOH preparations of specimens have up to 30% false-negative rates
[19,20,21,22].
Among filamentous fungi, representatives of the subdivision Ascomycotina
and Zygomycotina were isolated in decreasing order of frequency. The majority
of the fungi isolated in the present study have been previously reported
as etiologic agents of onychomycosis in different part of the world [23,24,25,26].
Baran et al. [9] reported the causal
significance of Fusarium spp. in proximal sub-ungual onychomycosis. In the
present study, members of the genus was represented by three species, Fusarium
oxysporum, Fusarum solani and F. moniliforme with F. moniliforme predominating.
There has been no previous reports of the presence of F. moniliforme in
onychomycosis in the literature. However, F. moniliforme has been reported
as the most frequent of Fusarium spp. isolated in corn at different stages
of development in the field and at harvest time and storage, being a soil-borne
fungus [27,28]
and in human food stuffs [29]. It is not
surprising therefore that it was one of the prevalent mycoflora found in
toenails of farmers in the villages studied, since remains of maize plants
after harvesting are usually ploughed to form part of soil, which is in
contact with toenails. It is however difficult to determine the participatory
role of this species in onychomycosis. The species was recovered in pure
culture in 12 of cases of onychomycosis among the farmers and was recovered
in mixed culture with C. albicans and T. rubrum in 4 and 3 cases respectively.
The occurrence of Candida species is not surprising. Among the yeasts, Candida
spp. has been reported in the literature as the etiologic agent of onychomycosis
all over the world [30]. Proximal sub-ungual
Candida onychomycosis had been reported [9,31,32].
Lim et al [6] had earlier reported
that 39% of the onychomycosis in Singapore were caused by Candida species.
Results obtained in this study follow a similar pattern. Among the dermatophytes,
T. rubrum had been previously reported to be the most common dermatophytes
associated with onychomycosis [23,33].
T. rubrum has been reported to have great capacity to colonize the hard
keratin of nails [34]. In the present investigation,
while T. rubrum was the most prevalent dermatophyte isolated, T. mentagrophytes
and Microsporum gypseum were also recovered. T. mentagrophytes ranked second
as the most prevalent dermatophyte isolated from the toenails. It also occurred
in some cases in mixed culture with Candida albicans (9 cases) and Penicillium
spp. (4 cases). The occurrence of the dermatophyte, M. gypseum (a keratinolytic
fungus) in low frequency is expected. Although it is a geophilic species
whose natural habitat is soil because of its saprophytic nature, it has
also been reported to cause infections in the keratinous tissues of humans
as well as domestic and wild animals [35]
but has not been associated with onychomycosis. In this study, the most
frequent clinical presentation was DLSO, a finding which is in consonance
with earlier reports [36]. The result of
T. rubrum being the main etiologic agent recovered from DLSO followed by
Candida albicans agreed with the reports of Alvarez et al. [37]
and Veer et al. [38]. The results
of the present study showed that T. mentagrophytes var. interdigitale was
the most prevalent dermatophyte in WSO, which is in accordance with reports
by Khosravi et al. [39] and Gupta
et al. [40]. Fusarium spp. (F. oxysporum
and F. solani) and A. terreus were also isolated in some cases of WSO. In
proximal sub-ungual onychomycosis (PSO) without paronychia, C. albicans
was the pathogen isolated in most cases (60%); in addition C. tropicalis,
F.moniliforme and A. niger were recovered in 20%, 15%, and 5% of PSO cases
respectively. One case of PSO was associated with F. solani. T. rubrum was
the predominant isolate in 9 of the 16 cases of total dystrophic onychomycosis
(TDO), with C. albicans and C. tropicalis in 4 and 3 cases respectively.
Since all the subjects in this investigation were males, it was impossible
to validate the assertion that onychomycosis is more common in females than
males as reported in many literature. In this study the majority of the
onychomycosis cases affected farmers of the age group of 40-49 years, an
observation which agreed with that of Bokhari et al. [41].
However there are conflicting reports on the age group affected in literature.
The similarity in most clinical presentations for onychomycosis caused by
moulds and dermatophytes makes it ultimately imperative to prepare cultures
of causal agents for identification before any treatment could be prescribed.
In all cases examined, lesions were observed mainly in nails of the big
toes. Total nail destruction was seen in 4 cases of DLSO. The locations
of study are rural areas where the presence of orthodox medicine is remote.
Therefore most cases were treated with herbal therapy which was said to
be effective (personal communication). Greer [15]
reported that 10-50% of onychomycosis in Nigeria are caused by moulds. It
is believed that the hot humid tropical and subtropical climate may be a
factor responsible for the causative role of moulds. It is desirable to
determine the prevalence of etiologic agents of onychomycosis in any particular
area because of the geographical differences in the etiology and epidemiology
of the disease in order to develop adequate control measures. Although most
moulds such as Penicillium and Fusarium isolated in this study are part
of the soil mycobiota involved in decomposition of dead plants materials
and foliage, they have been reported as pathogens in immunocompromised patients.
Other non-dermatophyte moulds species such as Scopulariopsis spp., Scytalidium
spp. Onychocola canadensis, Acremonium spp., which had been reported by
other investigators have not been identified in the present investigation.
The precise identification of the unidentified isolates in the study is
being carried out to foreclose this.
Acknowledgements
Thanks are due to Chief A. Fakunle of Kole-Aroro, who made contact and
accessibility to farmers and consent to collect specimens possible.
References
1. Zaias N. The nail in health and disease. 2nd
edition , Appleton and Lange, Norwalk,1990.
2. Faergemann J. The role of yeast in onychomycosis. Mycoses
1996; 39: 223- 224.
3. Ghannoum MA, Hajjah RA, Scher A. A large scale North
American study of fungal isolates from nails: the frequency of onychomycosis,
fungal distribution and antifungal susceptibility patterns. J Am Acad Dermatol
2006; 43: 641- 648.
4. Murray SC, Dawber RP. Onychomycosis of toenail: orthopaedic
and paediatric consideration. Australas J Dermatol 2002; 43:105-112.
5. Sobera J, Elewski B. Onychomycosis. In: Scher R.K, Daniel,
C.R (editors). Nails:diagnosis, therapy and surgery . 3rd edition, Elsevier
Saunders, 2005.
6. Lim JTE, Hock CC, Chee LG. Dematophytes and non-dematophytes
onychomycosis in Singapore. Australas J Dermatol 33:159:163, 1992.
7. Elewski BE. Onychomycosis: pathongensis, diagnosis and
management. Clin Microbiol Rev 1998; 11:415-429.
8. Sigler L, Congly H. Toenail infection caused by Onychocola
canadensis gen.et sp.nov. J Med Vet Mycol 1990; 28: 405- 417.
9. Baran R, Tosti A, Piraccin BM. Uncommon clinical patterns
of Fusarium nail infection: report of three case. British J Dermatol 1997;
136: 424- 427.
10. Gupta AK, Horgan- Bell CB, Summerbell RC. Onychomycosis
associated with Onychocola canadensis: ten case reports and a review of
the literature. J Am Acad Dermatol 1998; 39:410- 417.
11. Gupta AK, Ryder JE, Baran R, Summerbell RC. Non-dermagtophyte
onycomychosis. Dermatol Clin 2003; 21: 257- 268.
12. Menotti J, Machouart M, Benderdouche M, Cetre - Sossah
C, Morel P, Dubertret L. Polymerase chain reaction for diagnosis of dermatophyte
and Scytalidium spp. onychomycosis. Br J Dematol 2004; 151: 518- 519.
13. Summerbell RC, Copper E, Bunn U, Jamieson F, Gupta
AK. Onychomycosis: a critical study of technique, and criteria for confirming
the etiologic significance of non-dermatophytes. Med Mycol 2005; 43:39-
59.
14. Gugnani HC, Nzelibe FK, Osunkwo IC. Onychomycosis due
to Hendersonula toruloidea in Nigeria. J Med Vet Mycol 1986; 24: 239- 241.
15. Greer DL. Evolving role of nondermatophytes in onychomycosis.
Int J Dermatol 1995; 34: 521- 524.
16. Al-Doory, Y. Laboratory Medical Mycology Lea and Febiger,
Philadelphia, 1980.
17. Klich MA, Pitt JI. A laboratory guide to common Aspergillus
species and their teleomorphs. Commonwealth Scientific and Industrial Research,
1994.
18. Nelson PE, Toussoun TA, Marasas WFO. Fusarium spp:
An Illustrated Manual for Identification. The Pennsylvanica State Univ.
Press, 1983.
19. Haldane DJ, Robert E. A comparison of calcoflour white,
potassium hydroxide, and culture for the laboratory diagnosis of superficial
fungal infection. Diagn Microbial Infect Dis 1990; 13: 337-339.
20. Baran R, Chabasse D, Feulihade de chaurin M. Les onychomycosis.
II - Approche diagnostique. J Mycol Med 2001; 11: 5- 13.
21. Carballo MG, Roderguez NA, Peralta NB, de Cabalier
ED. Application of direct flourecence technique in the diagnosis of superficial
mycosis. Rev Fac Cien Med Univ Nac Cordoba 2002; 59: 57- 61.
22. Weinberg JM, Koestenblatt EK, Tutrone WD, Tishler HR,
Najarian L. Comparison of diagnostic methods in the evaluation of onychomycosis.
J Am Acad Dermatol 2003; 49: 193- 197.
23. Basnerjee U, Seth M, Pasricha JS. Study of onychomycosis
in India. Mycoses 1990; 33: 411- 415.
24. Brilhante RSN, Cordeiro RA, Medrano DJA. Onychmycosis
in Ceara (Northeast Brazil). Epidemiological and laboratory aspects. Men
Inst Oswaldo Cruz 2005; 100: 131- 135.
25. Dogra S, Kumar B, Bhansali A, Chakrabarty A. Epidemiology
of onychomycosis in patients with diabetic mellitus in India. Int J Dermatol
2002; 41: 647- 65.
26. ElSayed F, Ammoury A, FeghalyHaybe RA, Dhaybi R. Onychomycosis
in Lebanon: a mycological survey of 772 patients. Mycoses 2006; 49: 216.
27. Chulze SN, Ramirez ML, Farnochi MC, Pascade M, Visconts
A, March G. Fusarium and Fumonisins occurrences in Argentinian corn at different
ear maturity stages. J Agric Food Chem 1996; 44: 2297- 2307.
28. Farnochi MC, Etcheverry M, Dakero A, Chuzle S. Fusarium
(section Liseola) and fumonisins in storage corn from Argentina. Cereal
Res Communic 1997; 25: 587- 589.
29. Bullerman LLB, Tsai WJ. Incidence and level of Fusarium
proliferatum and fumonisins in corn and Corn -based foods. J Food Prot 1994;
57: 541-546.
30. Koussidou T, Devliotou-Panagiotidou D, Karakatsanis
G, Minas A, Mourellou O, Samara K. Onychomycosis in Nothern Greece during
1994-1998. Mycoses 2002; 45: 29- 37.
31. Dorko E, Jautova J, Tkacivoka L, Wantroubova A. The
Frequency of Candida species in onychomycosis. Folia Microbiol (Praha) 2002;
47: 727-731.
32. Ellabib MS, Agaj M, Khalifa Z, Kavanagh K. Yeasts of
the genus Candida are the dominant cause of onychomycosis in Libyan women
but not men: result of a 2-year surveillance study. Br J Dermatol 2002;
146: 1038- 1041.
33. Midgley G, Moore MK, Cook JC, Phan QG. Mycology of
nail disorders. J Am Acad Dermatol 1994; 31: 568- 574.
34. Hay RJ. Onychomycosis. Dermatol Clin 1993; 11(1): 167.
35. Dermage C, Contet-Audonneau N, Kombila M, Miegevlle
M, Berthonmeau M, Devroey C, Percebois G. Microsporum gypseum complex in
man and animals. J Med Vet Mycol 1992; 30: 301- 308.
36. Romano C, Gianni C, Difonzo EM. Retrospective study
of onychomycosis in Italy: 1985-2000. Mycoses 2005; 48: 42- 44.
37. Alvarez MI, Gonzalez LA, Castro LA. Onychomycosis in
Cali, Colombia. Mycopathologia 2004; 158: 181- 186.
38. Veer P, Pawardhan NS, Damle AS. Study of onychomycosis:
prevailing fungi and pattern of infection. Indian J Med Microbiol 2007;
25: 53- 56.
39. Khosravi AR, Aghamirian MR, Mahmoudi M. Dermatophytoses
in Iran. Mycoses 1994; 37: 43- 48.
40. Gupta AK, Ryder JE, Summerbell RC. Onychomycosis: classification
and diagnosis. J Drugs Dermatol 2004; 3: 51- 56.
41. Bokhari MA, Hussain I, Jahangir M, Haroon TS, Aman
S, Khurshid K. Onychomycosis in Lahore, Pakistan. Int J Dermatol 1999; 38:
591- 595.© 2011 Egyptian Dermatology Online
Journal
|

