Abstract:
Hemangiomas are the most common soft-tissue tumors of infancy, occurring
in approximately 5 to 10 percent of one-year-old children. There is no universally
accepted model for the etiology of hemangiomas, but there are many theories
about the origin of hemangiomas. The purpose of this study was to describe
the role of endoglin in hemangioma.
Materials and Methods:
We used human endoglin/CD105 Enzyme linked immunosorbent assay (ELISA)
kit to examine serum endoglin concentrations of 30 patients with hemangioma.
Result: The serum endoglin concentrations of patients with hemangioma
were significantly higher than control group.
Conclusion: Endoglin may have a role in the pathogenesis of hemangioma.
Keywords: Hemangioma, Enzyme linked immunosorbent assay, Endoglin
Introduction
Hemangiomas are the most common soft-tissue tumors of infancy, occurring
in approximately 5 to 10 percent of one-year-old children, Mulliken and
Glowacki [1,2]defined
hemangiomas as vascular tumors with a growth phase, marked by endothelial
proliferation and hypercellularity, and an involutional phase marked by
decrease in cellularity.
The incidence of hemangiomas is 3 times higher in female than male Infants.
There is also an increased occurrence in premature infants. Hemangiomas
usually appear soon after birth (though up to 30% may be present at birth),
typically proliferate during the first year of life and then involute during
the childhood years (up to 12 years). The terms capillary and cavernous
hemangioma are out of date and the lesions are more appropriately described
according to the depth of the lesion as superficial, deep, and compound
hemangioma. Superficial hemangiomas originate from the papillary dermis
and present as bright red macular or papular masses (previously called capillary
or strawberry hemangioma). Deep hemangiomas originate from the reticular
dermis or subcutaneous tissues and appear as bluish or relatively colorless
masses (previously called cavernous hemangioma). Compound hemangiomas have
superficial and deep components and were previously called capillary cavernous
hemangiomas. Hemangioma can be complicated by ulceration, infection, hemorrhage
and residual deformity. [1,3,4]
The histological features are dependent on the stage of the lesion, in
the proliferative phase, the lesion is highly cellular and contains plump
proliferating endothelial cells and pericytes, with a high mitotic activity
and numerous mast cells, vascular channels are not prominent. In the involutive
phase, the endothelial cells are flattened, the cell turnover is normal
and there are few mast cells, vascular channels filled with blood cells
predominate, and the lesion is eventually replaced by fibrofatty tissue.
[1]
There's no universally accepted model for the etiology of hemangiomas,
but there are many theories about the origin of hemangiomas. It has been
suggested that some hemangiomas originate from a first-trimester developmental
error in vasculogenesis, others says that the increased incidence of hemangiomas
in children born to women post-chorionic villus sampling suggests hemangiomas
are of placental origin. In this sampling procedure, a fetal trophoblast
sample is surgically removed from the placenta, leading to placental embolization
and maternal fetal transfusion. Some studies consider that underdeveloped
vasculature in the embryo could also result in vascular endothelial nests
that proliferate faster after birth than normal blood vessels. Others consider
that rapid tumor growth in the postnatal phase is possibly a result of the
loss of angiogenic inhibitors from the placenta or mother. Others suggesting
that the abnormal growth seen in proliferating hemangiomas are related more
to the local release of growth factors than to an altered endothelial phenotype.[5]
Today, the following treatments are used for hemangiomas: corticosteroids,
intralesional interferon alpha, imiquimod, vincristine, variety of lasers,
debulking surgery and watchful waiting. These treatments can be administered
individually or in combination.[3]
Endoglin is an auxiliary receptor for Transforming growth factor beta
(TGF-β) involved in the interaction with type II receptors for both TGF-β
and activin. Endoglin counteracts the inhibitory effect of TGF-β on cell
proliferation in several cell types including endothelial cells In this
respect, it is of interest that the inhibition of endoglin expression enhanced
the ability of TGF-β1 to suppress growth, migration and capacity to form
capillary tubes of cultured endothelial cells. In the absence of TGF-β1,
endoglin shows an anti-apoptotic effect in endothelial cells under hypoxic
stress, suggesting a protective role for endoglin against pro-apoptotic
factors. As endoglin directly interacts with a variety of TGF-β type I receptor,
this raises the possibility for additive or opposing effects of endoglin
on TGF-β receptor signaling. Thus, endoglin shows an inhibitory effect on
TGF-β / Activin receptor-like kinase 5(ALK5)/ Smooth muscle acting receptor3
(Smad3) cellular responses which block endothelial cell proliferation and
enhance extracellular matrix deposition. In addition, endoglin may be required
for TGF-β1/ALK1 signaling in some cell types, especially endothelial cells
inducing endothelial cell proliferation and degradation of extracellular
matrix. This balance between ALK5 and ALK1 may play a role in the regulation
of cell growth and differentiation in cells that express endoglin as well
as ALK1 and ALK5 . The mechanism by which endoglin potentiates TGF-β/ALK1
signaling appears to involve direct association of ALK1 with the cytosolic
and extracellular domains of endoglin, with the extracellular domain1 mediating
the enhancement of ALK1 signaling. These studies suggest that the functional
association of endoglin with ALK1 is critical for endothelial cell responses
to TGF-β.(6-10)
Patients And Methods
Patients
This study was done on 30 patients who attended the pediatric surgery
and dermatology outpatient clinics, Alexandria university hospitals and
proved by examination to have hemangiomas. Twelve patients had capillary
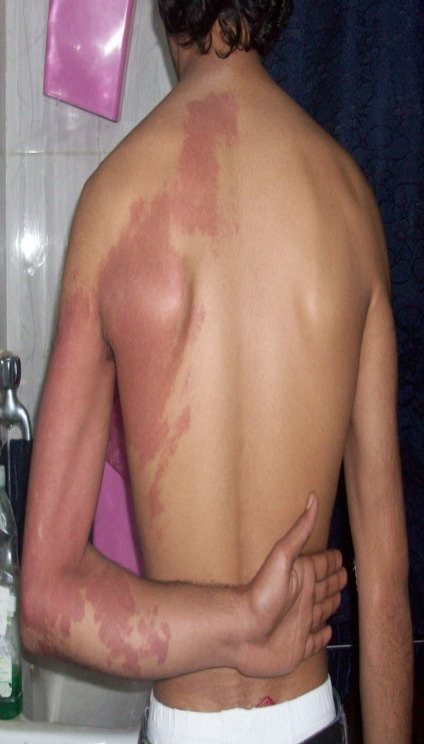
(superficial) hemangioma (Fig 1), 18 patients had cavernous hemangiomas
of which 9 patients had deep (Fig 2) and 9 patients had compound
type (Fig 3)
| Fig 1: Superficial hemangioma
(capillary). |
|
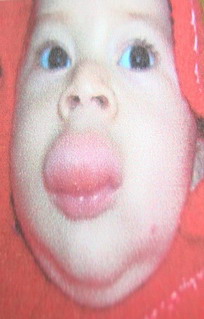
| Fig 2: Deep hemangioma (cavernous). |
|
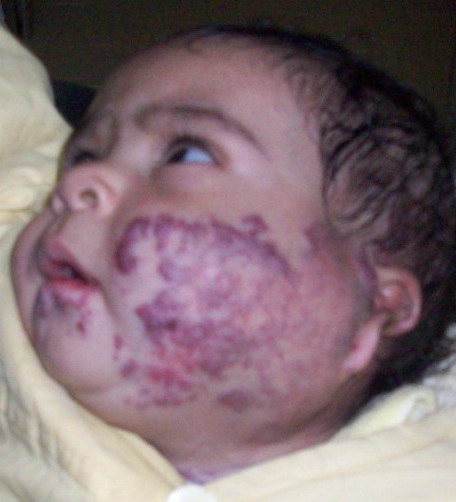
| Fig 3: Compound hemangioma (capillary
cavernous ). |
|
Twenty patients were females and 10 patients were males. Their age ranged
from 2 months up to 30 years.
The patients or their parents complaints were :
• Disfiguring patch in14 patients ,
• Non-painful swelling in 9 patients ,
• Painful swelling in 3 patients ,
• Bleeding lesion in 3 patients,
• Itchy lesion in one patient.
-The lesions dated since birth (0) up to 30 years with a mean duration
of 7.83 years and a median duration of 2 years.
- Hemangiomas were reported to be gradually increasing in size in 63.3%,
stationary in (20.0%) and regressive in 16.7% of patients.
- Ten percent (10.0%) of the parents of the studied patients were married
to relatives and 90.0% were non-consanguineous.
- The majority of cases (80.1%) had uncomplicated hemangiomas and 19.9%
reported complications such as bleeding, crustation or infection.
- Hemangiomas were located in the face in (36.7%), in the hand in 10.0%
and in the anterior chest wall or the scalp in 6.7% of patients. Others
sites, such as the arm, the abdominal wall, the labia majora or the buttock
were reported in 3.3%. Multiple locations of hemangiomas were encountered
in 26.7% of the studied patients.
- Hemangiomas measured from 0.5-40 cm. (mean= 4.810) in width and 0.5-40
cm. (mean=2 cm.) in length.
Control group: 60 subjects (age & sex matched with the patients with
hemangiomas) suffering from minor dermatological problems other than hemangioma
or admitted to pediatric surgical department for minor surgery (free from
hemangioma)
Venous blood sample were obtained from both groups after signing an informed
consent form (Informed consent was taken from the parents of children patients
participating in the study ), The study was approved by the Alexandria Faculty
of Medicine, Research Ethics Committee.
Methods
All patients were subjected to:
1-Complete history taking
2-Clinical examination
3-Photography:
The lesions were photographed by a Kodak camera with resolution 8 mega
pixel.
4-Investigation:
Venous blood samples (3-5 ml) were collected in tubes and allow them
to clot for 30 minutes before centrifugation for 15 minutes at approximately
1000 x g, then we remove serum and store samples at -20° C.
We used human endoglin/CD105 ELISA kit [11],
which includes the following reagents:
Endoglin microplate: 96 well polystyrene microplate (12 strips of 8 wells)
coated with a mouse monoclonal antibody against human endoglin.
Endoglin conjugate: 21 mL of mouse monoclonal antibody against human
endoglin conjugated to horseradish peroxidase with preservatives.
Endoglin standard: 100 ng of recombinant human endoglin in a buffered
protein base with preservatives.
Assay diluent: 11 mL of a buffered protein base with preservatives.
Calibrator diluent: 21 mL of a buffered protein base with preservatives.
Wash buffer concentrate: 21 mL of a 25-fold concentrated solution of
buffered surfactant with preservatives.
Color reagent A: 12.5 mL of stabilized hydrogen peroxide.
Color reagent B: 12.5 mL of stabilized chromogen (tetramethylbenzidine).
Stop solution: 6 mL of 2 N sulfuric acid.
Plate covers: 4 adhesive strips.
Procedure:
All reagents were brought to room temperature before its use then diluted
20 mL of wash buffer concentrate with distilled water to prepare 500 mL
of wash buffer.
Color reagents A and B were mixed together in an equal volumes then 200
micro liter of the resultant mixture was required per well.
Endoglin standard was reconstituted with 1.0 mL of distilled water. This
reconstitution produces a stock solution of 100 ng. /mL, and then mixed
the standard to ensure complete reconstitution and allowed the standard
to sit for a minimum of 15 minutes with gentle agitation prior to making
dilutions.
We pipetted 900 micro Liter of calibrator diluent into the 10 ng/mL tubes,
and then we pipette 500 micro Liter into the remaining tubes. We used the
stock solution to produce a dilution series. The 10 ng/mL standard serves
as the high standard and the calibrator diluent serve as the zero standard
(0 ng/ml). We added 100 micro Liter of assay diluent to each well and then
we added 50 micro Liter of standard control or sample per well and cover
it with the adhesive strip provided and incubated it for 2 hours at room
temperature on a horizontal orbital microplate shaker. A plate layout was
provided to record standards and samples assayed.
We aspirated each well and wash, repeating the process three times for
a total of four washes.
We wash by filling each well with wash buffer (400 micro liter) using
autowasher. Complete removal of liquid at each step is essential to good
performance. After the last wash, we removed any remaining wash buffer by
aspiration. We inverted the plate and blot it against clean paper towels.
Endoglin conjugate was added 200 micro L to each well and covering with
a new adhesive strip then incubated for 2 hours at room temperature on the
shaker.
We repeat the aspiration/wash as mentioned above.
Then we added 200 micro L of substrate solution to each well and incubated
for 30 minutes at room temperature on the benchtop with protection from
light.
We added 50 micro L of stop solution to each well. The color in the wells
changed from blue to yellow.
Determine the optical density of each well within 30 minutes, using a
microplate reader set to 450 nm
Data Processing and Analysis
After data collection, raw data was coded and scored and a coding instruction
manual was prepared. Data were fed to the computer using Epi-Info (version
3.0) and statistical analysis was performed using Statistical Package for
Social Sciences (SPSS version 15.0). Roc analysis was performed using Med
calc software. Significance of the obtained results was judged at the 5
% level.
1-Data processing:
Complete confidentiality was maintained while the data were being processed.
This stage had two major objectives:
1- Clean data by performing a series of comprehensive checks, making
corrections whenever possible. Different statistical procedures (frequencies,
means, standard deviations, median, and Interquartile Range (IQR) and cross
tabulations) were used to check the validity of data and spot any error.
2- Produce analytic results, which involved the recording of variables
into forms required for analysis.
2-Data analysis:
The following statistical measures were used:
1- Descriptive Statistics such as frequency, distribution, mean, median,
standard deviation and interquartile range to describe selected socio-demographic
and clinical profile of patients with hemangioma as well as their endoglin
level.
2- Bivariate analysis; student t test and One Way Anova (F) tests of
significance were used for statistical association between patients' profile
and their endoglin level.
3- Multivariate analysis was performed to assess the joint contribution
of selected socio-demographic and clinical variables (independent variables)
and endoglin level (Dependent variable). Variables included in the multiple
linear regression model were age (years), sex (male = 0, female = 1), onset
(other = 0, since birth = 1), type , location (others = 0, head = 1) , course
(others = 0, aggressive = 1), size (Others = 0, large = 1), complication
(no = 0, yes = 1), and type (others = 0, cavernous = 1).
4- ROC curve analysis was performed to evaluate the diagnostic accuracy
of endoglin level for the overall group of patients with hemangioma. A ROC
curve is a graphical representation of the tradeoffs between sensitivity
and specificity. ROC curve analysis was performed with use of data related
to endoglin level (ng/ml) from 30 hemangioma patients, and 60 controls.
Results
The comparison of endoglin level between cases of hemangioma and their
controls showed that The mean endoglin level for the cases was 8.99 1.55
ng/ml and this was significantly higher than that for their controls (7.13
2.21 ng/ml) where t = 4.961, and p = 0.000 .
The relation between endoglin level and selected demographic and clinical
factors.
Spearman correlation between age of the studied patients and their endoglin
level revealed a statistically significant negative correlation between
age of the patients and endoglin level (r = -0.348, p = 0.030). This means
the younger the age of patients with hemangioma is, the more increase in
endoglin level will be.
Also a statistically significant negative correlation between duration
of hemangioma and the endoglin level was found (r = - 0.356 and p = 0.027).
This means the shorter the duration of the lesion is the more increase in
the endoglin level will be. However, no statistical significant correlations
were noted between endoglin level and either onset or size of hemangioma
lesion (p = 0.104 and 0.080 respectively) .
In the present study despite that the endoglin level of male patients
was higher than that of female patients (9.40 1.78 versus 8.79 1.43 ng/ml),
yet this difference was not statistically significant (t = 1.017, p = 0.318)
.
Moreover, the association between endoglin level of the studied patients
and type of hemangioma was illustrated showing that the mean endoglin level
for patients with cavernous hemangioma (9.91 1.17 ng/ml) was higher than
that for those patients with capillary type (6.71 1.86 ng/ml) or that for
patients with combined hemangiomas (8.86 1.04 ng/ml). However, the difference
was not statistically significant (F = 2.771, p = 0.080).
Also, the mean endoglin level for patients with complications (9.74 0.57
ng/ml) was higher than that for those patients without complications (7.96
1.48 ng/ml). However, the difference was not statistically significant (t
= 2.015, p = 0.292).
ROC analysis
The diagnostic accuracy of endoglin level was evaluated for the overall
series using ROC curve analysis. A ROC curve is a graphical representation
of the tradeoffs between sensitivity and specificity. ROC curve analysis
was performed with use of data related to endoglin level (ng/ml) from 30
hemangioma patients, and 60 controls. The ROC curve analysis suggested that
the most useful cutoff value of serum endoglin concentration was the 8.4
ng/mL value, where the sum of sensitivity (73.33%) and specificity (70.00%)
was the highest (Fig 4 & Table 1).
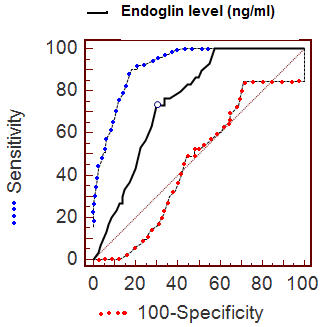
| Fig 4: ROC curve of serum endoglin
concentration for the diagnosis of hemangioma.
(Sensitivity plotted against 100 – specificity at
different concentrations of endoglin). |
|
|
Area under the ROC curve (AUC) |
0.754 |
|
Standard Error |
0.0418 |
|
95% Confidence Interval |
0.662 to 0.831 |
|
Z statistic |
5.543 |
|
Significance level P (Area=0.5) |
0.0001 |
Table 1 :Roc curve analysis of the studied sample (30 patients
& 60 controls)
Multivariate analysis
Predictors of elevated endoglin level
Multiple linear regression models were performed to predict the significant
contributing factors associated with elevated endoglin level among the studied
cases of hemangioma. After adjustment for confounders, higher level of endoglin
were significantly associated with younger age (b = - 2.823, p = 0.002),
female gender (b = 3.145, p = 0.005), since birth dating lesion (b = 2.657,
p = 0.002), and aggressive lesion (b = 3.525, p = 0.008). These variables
explained 79.639 % of the variability in elevated endoglin level (R2 = 79.639%)
indicating that the overall model was statistically significant where F
= 7.324, p = 0.007.
Discussion
In the present study among the thirty patients included there were 20
females (66.7%) and 10 males (33.3%) with a ratio of 2:1. In Anand et al
[12] their study was on 2398 patients the
male to female ratio 1:2.3. In Shapour et al [13]
their study was on 32 patients the female to male ratio 1.9:1. This was
more or less similar to literature where female predominance reached a ratio
of 3:1.
In the present study we measure the serum soluble endoglin level in 30
patients with hemangiomas by human endoglin/CD105 ELISA kit [11],
we found that the mean endoglin level for the cases was 8.99 1.55 ng / ml
which is significantly higher than their control(7.13 2.21 ng / ml) where
t=4.961 and p=0.000. As endoglin is an auxiliary receptor for TGF-β involved
in the interaction with type II receptors for both TGF-β and activin. Endoglin
counteracts the inhibitory effect of TGF-β on cell proliferation in several
cell types including endothelial cells. Many studies were done to prove
the inhibitory effect of TGF-β on Basic fibroblast growth factor (bFGF).
Andrew et al [14] found in their study
that TGF-β is a potent inhibitor of the proliferative activities of bFGF
on vascular and capillary endothelial cells; this inhibition is of non competitive
interaction and dose dependent. Mohammed et al [15]
found that TGF-β inhibits bFGF induced proliferation of cultured bovine
retinal endothelial capillary cells in dose dependent manner. Marijke et
al [16] their study shows that the antagonism
of fibroblast growth factor induced cell proliferation on bovine aortic
endothelial cells in culture is of non competitive manner and dose dependent.
Sakela et al [17] found that bFGF is potent
inducer of angiogenesis in vivo and stimulate the production of both urokinase
and tissue type plasminogen activators (PAs) in cultured bovine capillary
endothelial cells and this effect is diminished by pictogram amount of TGF-β,
so TGF-β has opposing effect on bFGF induced proliferation on vascular and
capillary endothelial cells. bFGF is critical to angiogenesis and this was
proved by Zahng Rong et al[1] who studied
the concentration of bFGF in 25 patients with proliferating and 14 patients
with involuting hemangiomas and he found that the serum bFGF concentration
of proliferating hemangioma were significantly higher than those of involuting
one.
From all the above information we can conclude that endoglin inhibits
TGF-β which inhibits bFGF, so endoglin indirectly activate bFGF leading
to vascular and capillary endothelial cell proliferation. This may prove
that our result can be documented. To our knowledge there is no similar
study to ours to date.
In the present study we found that there is a statistically significant
negative correlation between age of the patient and endoglin level showed
by spearman correlation r=-0.348 and p=0.030 which means that the younger
the age of the patient, the higher the endoglin level and this is coincident
with the nature of hemangioma that is proliferative in early phases and
involutive in late phases. Michelle et al [19]
reported in his study on hereditary hemorrhagic telangiectasia (HHT) type1
that there is inverse correlation between age and level of plasma endoglin
indicating that age is effect modifier highest in children and decrease
progressively with age as the levels of plasma endoglin measured by ELIZA
in 197 individuals with and without HHT show inverse correlation with age
r=-0.22 and p<0.002.
As regard the duration of hemangioma and endoglin level there is negative
correlation as r=-0.356 and p=0.027 which means that the shorter the duration
of the lesion the more the endoglin concentration. All the above results
can prove that endoglin has a role in hemangioma as hemangioma starting
proliferative in early phases where we found endoglin concentration is high
and becoming involutive in late phases where we found endoglin concentration
is decreasing.
From the result of our study (to our knowledge this is the first study
to be done on the role of endoglin in hemangioma); higher level of endoglin
were significantly associated with younger age, female gender, lesion dating
since birth, and aggressive lesion.
Conclusion
Endoglin may have a role in the pathogenesis of hemangioma through its
involvement in TGF-β signaling during developmental angiogenesis.
References
1. Muliken JB, Glowacki J. Hemangiomas and vascular malformations
in infants and children: a classification based on endothelial characteristics.
Plast Reconstr Surg 1982; 69: 412-22.
2. Enjolras O, Muliken JB. Vascular tumors and vascular
malformation. Adv Dermatol 1997; 13: 375-422.
3. Brukner AL, Friden IJ. Hemangioma of infancy. J Am Acad
Dermatol 2003; 48: 477-93.
4. Drolet BA, Esterly NB, Frieden IJ. Hemangioma in children,
N Engl J Med 1999; 34: 173-81.
5. Constantin G, Maurice A. The pathogenesis of hemangiomas:
A Review. Plast Reconst Surg 2006; 117: 29-35.
6. Cheifetz S, Bellon T, CaLes C, Vera S, et al. Endoglin
is component of transforming growth factor beta receptor system in human
endothelial cells. J Biol chem. 1992; 267: 19027-30.
7. Lastre P, Letamendia A, Zhang H, Rius C, et al. Endoglin
Modulates cellular responses to TGF-β l J Cell Biol. 1996; 133:1109-21.
8. Gougos A, ST Jacaues S, Greaves A, et al. Identification
of distinct epitopes of endoglin, an RGD- containing glycoprotein of endothelia
cells, leukemic cells and syncytiotrophoblasts. Int Immunol. 1992; 4: 83-92.
9. Carvalho RL, Jonker L, Goumans MJ, Larsson J et al. Defective
paracrine signallign by TGF-β in yolk sac vasculature of endoglin mutant
mice: A paradigm for HHT Development. 2004; 131: 6237-47.
10. Santibenz JF, letamendin A, Perez- Barriocanal F, et
al. Endoglin increases eNOs expression by modutaling Smad2 protein levels
and Smad2- dependent TGF-β signaling. J cell physiol 2007; 210: 456-68.
11. Alberto O, Alicia RB, Miguel P, Lorena B, et al. Human
recombinant erythropoietic agents do not induce changes in circulating levels
of endoglin and vascular endothelial growth factor in anaemic cancer patients.
Cancer letters 2007; 255: 71-6.
12. Anand P, Ajay Narayan G, Saroj CG, et al; Twenty year's
experience of steroids in infantile hemangioma a developing country's perspective.
pediatr surg 2009; 44:688-94.
13. Shapour O, et al. intralesional bleomycin injection
for treatment of hemangiomas. Dermatol surg 2005; 31: 499-501.
14. Andrew Baird and Terri Durkin. Inhibition of endothelial
cell proliferation by type beta-TGF interactions with acidic and basic fibroblast
growth factors. Biochem Biophys Res communications 1986; 138: 476-82.
15. Mohammed B, Francois M, et al. Opossing effect of bFGF
and TGF-β on the proliferation of cultured bovine retinal capillary endothelial
cells. EXP Eye Res 1989; 48: 791-99.
16. Markijke FS, Gertraud M, et al Transforming growth
Factor beta inhibits endothelia cell proliferation. Biochem Biophys Res
comm 1986; 137: 295-302.
17. 17. Sakela O, Moscatelli D, et al. the opposing effect
of bFGF and TGF-β on the regulation of plasminogen activator activity in
capillary endothelial cells. JCB 1987; 105: 957-83.
18. ZHANG Rong-ming,WANG Hong-mei,WANG Ping, ZHAO Li-yan,ZHANG
Jing. Detection and clinical value of bFGF in the serum of hemangioma infants.
Journal of China Medical University 2009-09 http://en.cnki.com.cn/Article_en/CJFDTotal-ZGYK200909019.htm.
19. Michelle L, Merry-Lynn MD, et al. Reduced endothelial
secretion and plasma levels of TGF beta1 in patients with hereditary hemorrhagic
telangiectasia. OXF J Med Cardiovas Res 2005; 68: 155-64.
© 2011 Egyptian Dermatology Online Journal
|




