|
|
Background:
Psoriasis, an inflammatory disease of skin, shares many immunological features
with other complex disorders such as cardiovascular disease, metabolic syndrome
and depression. Metabolic syndrome is a cluster of risk factors including
central obesity, atherogenic dyslipidaemia, hypertension and glucose intolerance.
It is a strong predictor of cardiovascular diseases, diabetes and stroke.
Aim:
To investigate the prevalence of metabolic syndrome in patients with psoriasis.
Methods:
It was a prospective, hospital based case-control study of 60 patients with
moderate to severe psoriasis having BSAI>10% and PASI>10 and 30 age matched
controls having minor skin ailments. Venous samples were taken at the enrolment
visit after the subjects had fasted overnight (at least 8 h). Serum cholesterol
and triglycerides were measured with enzymatic procedures. Plasma glucose
was measured using a glucose oxidase method. Metabolic syndrome was diagnosed
by the presence of three or more criteria of the modified version of National
Cholesterol Education Programme's Adult Panel III (ATP III). Statistical
analysis of the data was done using statistical processing software (SPSS-17).
Results:
Psoriatic patients had mean disease duration of 8.16±8.30 years, mean BSAI
was 41.88±15.803, mean PASI score was 17.606±6.831 and mean onset of age
of psoriasis was 31.41±13.415. Metabolic syndrome was significantly more
common in psoriatic patients than in controls [15 (25%) vs 1 (3.3%), odds
ratio (OR) = 9.667, P<0.05]. Psoriatic patients also had a significantly
higher prevalence of hypertriglyceridemia [26/60 (43.3%) vs. 4/30 (13.3%)
odds ratio (OR) = 4.971; P<0.01] and arterial hypertension [28/60 (46.6%)
vs. 4/30 (13.3%); P<0.01].
Conclusion:
Psoriatic patients have a high prevalence of metabolic syndrome which can
favour cardiovascular events. Psoriatic patients should be encouraged to
correct aggressively their modifiable cardiovascular risk factors including
metabolic syndrome.
Introduction
Psoriasis is a chronic inflammatory skin disease that affects 1-3% of the
population.[1,2] Epidemiological research
has shown that hypertension, heart failure and diabetes are significantly
more common in patients with psoriasis than in controls.[3,4]Similarities
also exist among psoriasis, the metabolic syndrome and atherosclerosis,
with all three conditions characterized by an inflammatory process driven
by Th1 cytokines.[5,6] Metabolic syndrome
is a cluster of risk factors including central obesity, atherogenic dyslipidaemia,
hypertension and glucose intolerance. (Fig 1) It is a strong predictor
of cardiovascular diseases, diabetes and stroke
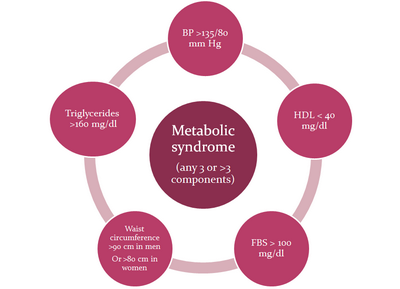
| Fig 1: Various components of metabolic
syndrome. |
|
Methods
The present
study comprised of clinically diagnosed patients of psoriasis taken from
Department of Dermatology, Govt. Medical College, Amritsar (INDIA).
The following
categories of patients were taken at random for the study:
1) Group I
(Study Group): 60 clinically and histopathologically diagnosed patients
of moderate to severe psoriasis having PASI score > 10 and body surface
area index (BSAI) more than 10%.
2) Group II
(Control Group): 30 patients attending Dermatology department with various
minor ailments (age matched).
The source
population for cases and controls was the same. An informed consent was
taken from all patients and patient characteristics were recorded on a standard
proforma. Statistical analysis of the data was done using statistical processing
software (SPSS-17). Relevant data included age, gender, weight, height,
body mass index, waist circumference, blood pressure, smoking habit, age
of onset and duration of psoriasis, type and severity of psoriasis. Body
mass index (BMI) was calculated as weight in kilograms/height2
in meters.[7] To determine waist circumference, we located
the upper hip bone and placed the measuring tape at the level of the upper
most part of the hip bone around the abdomen (ensuing the tape measure was
horizontal). The tape measure was snug but did not cause compression on
the skin. Blood pressure was recorded as the average of two measurements
after subjects have been sitting for five minutes. Severity of psoriasis
was assessed according to psoriasis area and severity index (PASI) and percent
body surface area (%BSA) involvement. History of smoking and alcoholism
was taken. The current smokers were considered to be those patients who
had been smoking five or more cigarettes per day for minimum of five years.
Patients consuming five or more drinks per day for minimum of five years
were considered to be alcoholic in the present study. Metabolic syndrome
was diagnosed by the presence of three or more of five criteria of the modified
version of National Cholesterol Education Programs Adult Panel III (ATP
III) [waist circumference >90 cm in men or >80 cm in women; hypertriglyceridemia
>160 mg/dl; high density lipoprotein (HDL) cholesterol < 40 mg/dl; blood
pressure >130/85 mmHg; fasting plasma glucose of > 100 mg/dl.[8]
Venous samples were taken after the patients had fasted overnight (at least
8 hours). Serum cholesterol and triglycerides were measured with enzymatic
procedures. Plasma glucose was measured using a glucose oxidase method.
Results
The descriptive
characteristics of study and control group are given in (Table 1). In the
present study, majority of the psoriatic patients, i.e. 46 (76.6%) had PASI
score 10 to 20 and 14 (23.2%) patients had PASI score of >20 including 4
(6.6%) patients with PASI >30 (Mean ±SD = 17.606±6.831) while 55 (91.7%)
patients had more than 20% body surface area involvement and only 5 (8.3%)
had Body Surface Area Involvement <20% (Mean ±SD = 41.88±15.803) (Fig.
2 and 3).
|
Demographic Features |
Study Patients (n=60) |
Control Patients (n=30) |
|
|
No. of patients |
No. of patients |
|
Gender |
|
Male |
44 |
22 |
|
Female |
16 |
8 |
|
Rural/Urban Distribution |
|
Rural |
37 |
15 |
|
Urban |
23 |
15 |
|
Age (in years) |
|
<30 |
22 |
11 |
|
31-50 |
23 |
12 |
|
>51 |
15 |
7 |
|
0ccupational Status |
|
Student |
4 |
5 |
|
Homemaker |
14 |
7 |
|
Labourer |
15 |
3 |
|
Farmer |
13 |
4 |
|
Businessman |
3 |
2 |
|
Employees |
8 |
9 |
|
Retired personnel |
3 |
- |
Table 1: Descriptive
characteristics of study group and controls
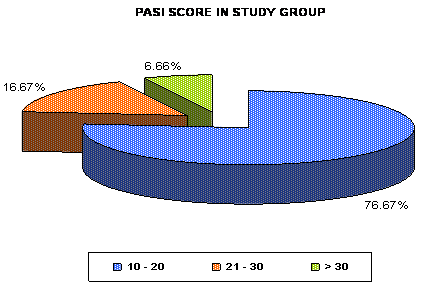
| Fig 2: Distribution of number of
psoriatic patients (in percentage) according to
Psoriasis Area Severity Index (PASI) score in study
group. |
|
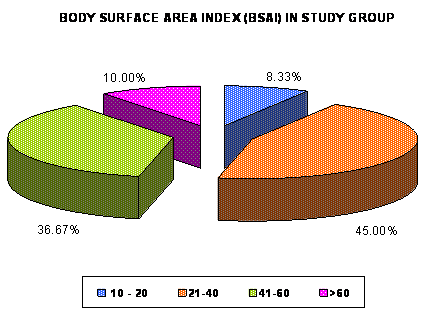
| Fig 3: Distribution of number of
psoriatic patients (in percentage) according to Body
Surface Area index (BSAI) in study group. |
|
Maximum number
of patients i.e. 17 (28.3%) were affected by the disease from 1-5 years
while in only 6 (10%) patients, the duration of disease was >20 years and
the duration of disease ranged from 20 days to 32 years (Mean ±SD = 8.16±8.30).
Minimum age of onset of disease in this study was 12 years and maximum age
of onset was 60 years (Mean ±SD = 31.41±13.415).
While comparing
risk factors in psoriasis patients and controls, 9 (15%) psoriatic patients
were smokers while none was smoker in the control group and this difference
was statistically significant (p-value<0.05). 23 (38.3%) patients in the
study group were alcoholic as compared to only 2 (6.6%) patients of control
group. This difference in two groups was also statistically significant
[OR 5.595 (95% CI, 1.608-19.153), p-value<0.01]. (15/60= 25%) of psoriatic
patients were found to have higher prevalence of metabolic syndrome as compared
to controls i.e. 01/30 (3.3%) [OR= 9.667 (95% CI, 1.526 – 59.609), p< 0.05].
Individual components of metabolic syndrome like hypertriglyceredaemia and
hypertension were also more prevalent in psoriasis patients than in controls.
Hypertension was present in 28 (46.6%) patients in the study group as compared
to 4 (13.3%) patients in control group [OR 5.688 (95% CI, 1.833-17.428),
p-value<0.01]. Hypertriglyceridaemia was present in 26 (43.3%) patients
in study group as compared to 4 (13.3%) patients in control group [OR 4.971
(95% CI, 1.60-15.248), p-value<0.01]. Decreased levels of HDL were present
in 2 (3.3%) patients in study group as compared to none in control group.
While comparing obesity by Body Mass Index (BMI), 32 (53.3%) psoriatic patients
were obese as compared to 10 (16.6%) controls (p>0.05). By Waist Hip Ratio
(WHR) criteria, 17 (28.3%) males and 13 (21.3%) females were obese in study
group as compared to 4 (13.3%) males and 4 (13.3%) females in controls (p>0.05).
By Waist Circumference (WC) criteria, 21 (35%) males in study group while
in control group 6 (20%) males were obese (p>0.05) while only 14 (23.3%)
females in study group and 4 (13.3%) females in controls were found to be
obese (p-value<0.05). The prevalence of various components of metabolic
syndrome in psoriatic cases and controls along with odds ratio and P value
are given in [Table2].
|
Components of metabolic syndrome |
Study group(n) |
Controls(n) |
Odd’s ratio (OR) |
P-value (Significance) |
|
Risk Factors |
Smokers |
9 |
- |
|
0.025 |
|
Alcoholic |
23 |
3 |
OR=5.595 |
0.002 |
|
No. of obese patients as per obesity indices |
|
Body Mass Index (BMI) |
32 |
10 |
|
0.279 |
|
Waist HIP Ratio (WHR) |
|
|
|
|
|
M |
17 |
4 |
|
|
|
F |
13 |
4 |
|
0.092 / 0.112 |
|
|
|
|
|
|
|
Waist Circumference (WC) M |
21 |
6 |
|
0.11 / 0.045 |
|
F |
14 |
4 |
|
|
Hypertension (>130/85 mm of Hg) |
28 |
4 |
OR=5.688 |
0.002 |
|
Hypertriglyceridemia (>160mg/dl) |
26 |
4 |
OR=4.971 |
0.005 |
|
Low Level HDL (<40mg/dl) |
2 |
- |
|
0.312 |
|
Diabetes Mellitus (FBS>100mg/dl) |
5 |
1 |
OR=2.636 |
0.659 |
|
Metabolic Syndrome |
15 |
1 |
OR=9.667 |
0.017 |
P>0.05 Not-Significant; P<0.05 Significant at 5% significance;
P<0.01 Significant at 1% significance; n= Number of patients
Table 2: Distribution of risk factors and components of metabolic syndrome in
study group (n=60) and controls (n=30)Discussion
Recent advances in
our understanding of the role of inflammatory cells and mediators in the
pathogenesis of psoriasis have shifted the clinical perspective on psoriasis
from merely a skin disorder to that of a systemic inflammatory process which
has a direct bearing on the prevalence of other co-morbid conditions in
patient population.[9] Given the link between atherosclerosis
and inflammation, the risk of cardiovascular disease is likely to be increased
in patients with psoriasis.[10] Lifestyle factors, such
as smoking, increased alcohol consumption, diabetes, hypertension and obesity
may also contribute to the development of cardiovascular disease and increased
inflammation in these patients[11].
Similarities
exist among psoriasis, the metabolic syndrome and atherosclerosis, with
all three conditions characterized by an inflammatory process driven by Th1
cytokines.[5,6]
(Fig 4)
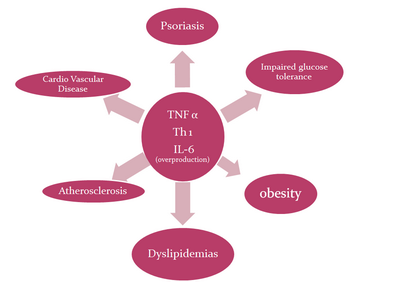 | Fig
4:
showing
pro-inflammatory cytokines such as TNF-α, Th1 and IL-6 driven
common pathway for pathogenesis of psoriasis and its
comorbidities. |
|
The metabolic
syndrome which comprises cluster of risk factors including obesity, dyslipidemia,
hypertension and glucose intolerance is a strong predictor of cardiovascular
disease conferring a cardiovascular risk greater than that for individual
components.[12] Pro-inflammatory cytokines such as TNF-α
and Th1 cytokines are overproduced in patients with psoriasis are likely
to contribute to the increased development of metabolic syndrome.[13]
In our study 15(25%) patients were found to be having Metabolic Syndrome
as compared to 1(3.3%) in control group which was statistically significant
(p<0.05). Similar results have been reported in other studies. Similar studies
by Nisa et al, Gisondi et al and Sommer et al also found high prevalence
of metabolic syndrome in psoriatic patients as compared to controls [13,14,15]
Association of psoriasis with individual components
of metabolic syndrome had been the focus of many cross-sectional studies
in the recent past. Several studies with varying population and analytical
approaches have found an association between psoriasis and increased prevalence
of diagnosis of dyslipidemia.[17, 18,
19] In addition, multiple studies have documented increased
prevalence of diabetes and hypertension in patients with psoriasis.[20,21,22]
There have been different observations in different studies [Table 3].
|
|
Nisa et al[14] |
Gisondi et al[15] |
Thomas et al[22] |
Present study |
|
Comorbidities |
Study patients vs Control Patients %age |
|
H/o smoking |
42% vs 10% |
36.2% vs 21% |
- |
15% vs 0% |
|
Hypertriglyceridemia |
48.6% vs 16% |
37.8% vs 23.3% |
4.10% |
43.3% vs 13.3% |
|
Hypertension |
49.3% vs 16% |
40.8% vs 39.5% |
14.10% |
46.6% vs 13.3% |
|
Low HDL levels |
56.6% vs 62.6% |
18% vs 21.5% |
- |
3.3% vs 0% |
|
Obesity (BMI) |
14.6% vs 20.6% |
57.1% vs 47.6% |
6.60% |
58.3% vs 33.3% |
|
Diabetes Mellitus |
18% vs 5.3% |
19.2% vs 20.9% |
11.60% |
8.3% vs 3.3% |
|
Metabolic Syndrome |
28% vs 6% |
30.1% vs 20.6% |
- |
25% vs 3.3% |
Table 3: Comparison of
prevalence of metabolic syndrome and its components in different studies
In our study,
28(46.6%) patients were hypertensive as compared to 4 (13.3%) among controls
(p<0.01). In one of the study by Henseler et al in 40,000 dermatological
patients, it was found that there was 1.9 fold greater likelihood of hypertension
in patients with psoriasis than the patients with other dermatological conditions.[3]
In another study by Kimhi O, it was found that patients of psoriasis had
a significantly higher incidence of hypertension than controls.[23]
Similar results were also seen in our study where patients with psoriasis
had 5.688 fold (CI 1.833-17.428) higher incidence of hypertension than controls.
In a study by Nisa et al, it was found that 49.3% patients were hypertensive
in study group while only 16% were hypertensive in control groups.[14]
In another study by Gisondi et al, 40.8% patients were hypertensive in study
group and almost equal numbers of patients (39.5%) were hypertensive in
control groups[15] while Thomas et al observed 14.1% psoriatic
patients to be hypertensive.[22]
Obesity is
a pro inflammatory state and the adipose tissue is a rich source of inflammatory
mediators as adipocytokines. Leptin, a protein hormone produced by adipose
tissue that plays a key role in regulating energy intake and expenditure,
is a stimulator of T cells, and in mouse models, leptin deficiency negates
autoimmune pathophysiology, suggesting a potential link between adipose
tissue and psoriatic inflammation.[12,23]
TNF α induces the rapid release of leptin from adipocytes in culture & causes
circulating leptin levels to increase in vivo.[25] A study
by Nisa et al showed a statistically insignificant difference among psoriatics
vs. controls. 14.6% of the psoriatic patients were obese as compared to
20.6% of controls. [14] Similar results were obtained
by others [15, 22]. In our study 58.3%
of patients were obese in study group as compared to 33.3% in control group
though our findings were statistically non-significant.
Numerous studies
have shown increased prevalence of smoking in patients with psoriasis as
compared with control. The association may be explained partly by the action
of nicotine in promoting Th1 mediated inflammation.[6]
Studies
have shown that cigarette smoking induces an overproduction of IL-1β
and increases the production of TNF-α and transforming growth factor-β,
which have been associated with psoriasis severity.[26]
In
our study, 15% of patients were smokers while in control group none were
smoker and the result was statistically significant (p<0.05),which were
consistent with findings of other studies [14,15]
Alcoholism
has been related with psoriasis. In our study 38.3% patients were alcoholic
as compared to 10% in controls. In another study by Naldi et al, 215 newly
diagnosed patients and 267 controls showed that risk for psoriasis was higher
in alcoholics than in non-alcoholics.[16] The increase
in risk for the development of psoriasis was 1.3-fold (95%CI, 0.8–2.3) for
patients who had one or two drinks per day and 1.6-fold (95% CI, 0.9–3.0)
for patients who had three or more drinks per day.[16]
Pro-inflammatory
cytokines like TNF-α and IL-6 which are over expressed in psoriasis are
known to contribute to dyslipidaemia [13]. Compared with
the control subjects, patients with psoriasis are more likely to have abnormal
lipid metabolism. Studies have demonstrated significantly higher level of
S. Cholesterol, triglycerides and LDL in psoriasis as compared to control
population. These patients also have significantly lower level of HDL than
controls.[4] Hypertriglyceridemia was seen in 43.3% cases
as compared to 13.3% in controls in our study, i.e. OR 4.971 (CI 1.60-15.248
p<0.01).[4] Similar results were seen in a study by Nisa
et al where 48.6% of psoriatic patients had hypertriglyceridemia as compared
to 16% in controls.[14] Study by Gisondi et al showed
37.8% of psoriatic patients were having hypertriglyceridemia as compared
to 23.3% in controls.[15] Low level of HDL was seen in
3% patients in study group and none in control group in our study. Abnormal
lipid profile has also been seen in our study but these results did not
reach statistical significance.
Pro-inflammatory
cytokines TNF-α and IL-6 are over expressed in psoriasis and known to contribute
to insulin resistance.[13] In an Italian study, 41.5%
patients had diabetes in psoriasis patients as compared to 24.3% in controls
(p=0.001).[15] In a study by Nisa et al, 18% psoriatic
patients were diabetic as compared to 5.3% in controls (p<0.001).[14]
Gisondi et al reported 19.2% psoriatic patients having diabetes as compared
to 20.9% in controls (p>0.05).[15] In our study,
incidence of diabetes though was found to be 8.3% in psoriasis patients
as compared to 3.3% in controls, but the results were not significant (p>0.05)
as those in the study by Gisondi et al.[15]
References
1. Schon MP, Boehncke W-H. Psoriasis. N Engl J Med. 2005;
352:1899-1912.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence
and treatment of psoriasis in the United Kingdom: a population-based study.
Arch Dermatol. 2005; 141:1537-1541.
3. Henseler T, Christophers E. Disease concomitance in psoriasis.
J Am Acad Dermatol. 1995; 32:982-986.
4. Mebazaa A, El Asmi M, Zidi W, et al. Metabolic syndrome
in Tunisian psoriatic patients: prevalence and determinants. J Eur Acad
Dermatol Venereol. 2011; 25:705-709.
5. International Psoriasis Council. Obesity in psoriasis:
the metabolic, clinical and therapeutic implications. Report of an interdisciplinary
conference and review. Br J Dermatol. 2007; 157:649-655.
6. Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease
in psoriasis. J Am Acad Dermatol. 2007; 57:347-354.
7. International Obesity taskforce. The Asia-Pacific prospective:
Redefining obesity and its treatment; Feb. 2000.(cited 2008 Dec 15) Availablefrom:
http://www.wpro.who.int/NR/rdonlyres/0A35147B-B1D5-45A6-9FF2-F7D86608A4DE/0/Redefiningobesity.pdf
8. National Heart, Lung, and Blood Institute. Diagnosis
and management of the metabolic syndrome: an American Heart Association/National
Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;
112:2735-2752.
9. Krueger JG, Bowcock A. Psoriasis pathophysiology: current
concepts of pathogenesis. An Rheum Dis. 2005; 64 Suppl 2:ii30-ii36.
10. Hansson GK. Inflammation, atherosclerosis, and coronary
artery disease. N Engl J Med. 2005; 352:1685-1695.
11. Weinberg JM. Lifestyle issues and psoriasis. Cutis.
2006; 78:160.
12. Prey S, Paul C, Bronsard V, et al. Cardiovascular risk
factors in patients with plaque psoriasis: a systematic review of epidemiological
studies. J Eur Acad Dermatol Venereol. 2010; 24 Suppl 2:23-30.
13. Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal
M. Increased prevalence of the metabolic syndrome in patients with moderate
to severe psoriasis. Arch Dermatol Res. 2006; 298:321-328.
14. Nisa N, Qazi MA. Prevalence of metabolic syndrome in
patients with psoriasis. Indian J Dermatol Venereol Leprol. 2010; 76:662-665.
15. Gisondi P, Tessari G, Conti A, et al. Prevalence of
metabolic syndrome in patients with psoriasis: a hospital-based case-control
study. Br J Dermatol. 2007; 157:68-73.
16. Naldi L, Parazzini F, Brevi A, et al. Family history,
smoking habits, alcohol consumption and risk of psoriasis. Br J Dermatol.
1992; 127:212-217.
17. Nevitt GJ, Hutchinson PE. Psoriasis in the community:
prevalence, severity and patients' beliefs and attitudes towards the disease.
Br J Dermatol. 1996; 135:533-537.
18. Gerdes S, Zahl VA, Knopf H, Weichenthal M, Mrowietz
U. Comedication related to comorbidities: a study in 1203 hospitalized patients
with severe psoriasis. Br J Dermatol. 2008; 159:1116-1123.
19. Farber EM, Carlsen RA. Psoriasis in childhood. Calif
Med. 1966; 105:415-420.
20. Buntin DM, Skinner RBJ, Rosenberg EW. Onset of psoriasis
at age 108. J Am Acad Dermatol. 1983; 9:276-277.
21. Kaur I, Handa S, Kumar B. Natural history of psoriasis:
a study from the Indian subcontinent. J Dermatol. 1997; 24:230-234.
22. Jayakar Thomas, Hok Kumar N Ashok, Manoharan D, Cynthia
S, Lva Prabu K Selva, Hwak Ahmed N Ashwak. A study of comorbid conditions
in psoriasis. J Pak Assoc Derma Oct - Dec 2009; 19(4):200-2.
23. Kimhi O, Caspi D, Bornstein NM, et al. Prevalence and
risk factors of atherosclerosis in patients with psoriatic arthritis. Semin
Arthritis Rheum. 2007; 36:203-209.
24. Ozturkcan S, Ermertcan AT, Sekuri C, Kylyccyoglu B.
Cardiovascular findings in patients with psoriasis. Ann Saudi Med. 2006;26:159-161.
25. Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW,
Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related
hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest.
1997; 100:2777-2782.
26. Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy
M. Comorbidities associated with psoriasis: an experience from the Middle
East. J Dermatol. 2010; 37:146-155.© 2011
Egyptian Dermatology Online Journal
|




