|
|
Abstract
Acral lentiginous melanoma (ALM) is a clinicopathologic variant of
malignant melanoma of the skin. It occurs in the acral or peripheral
parts of the limb, on the plantar or palmar surfaces of the hands and
feet, or the subungual areas of the fingers or toes. ALM is
histologically and clinically distinct from other types of melanoma like
nodular melanoma (NM), superficial spreading melanoma (SSM), and Lentigo
maligna melanoma (LMM). We report 3 cases of acral lentiginous melanoma
2 of which showed good prognosis after surgical excision.
Introduction
Acral lentiginous melanoma accounts for about 2%to3% of all
melanomas. Overall incidence of melanoma is less in dark skinned
individuals but ALM has higher incidence in dark races than other types
of melanoma. It is associated with a worse prognosis than cutaneous
malignant melanoma overall. Hispanic whites and Asian Pacific Islanders
have worse survival rates than other groups. Factors such as increased
tumor thickness and more advanced stage at presentation are the most
likely explanations. As this tumor involves functional parts, the
surgical margins are be compromised which may be responsible for the
recurrences. Hence early diagnosis and surgical excision is the key in
the management.
Cases report
Three males of which two aged 65 years and one aged 46 years
presented with complaints of asymptomatic black colored lesion on the
soles in the first two cases and on the right great toe in the third
case, since 3 months, 9 yrs and 2 yrs respectively. There was history of
trauma to the sole with glass particle in the first case while the other
two cases did not give history of any trauma prior to the appearance of
the lesion. All of them did not take any treatment in the past. There
was no history of irradiation to the local part or history of chronic
arsenic ingestion.
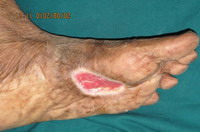
Cutaneous examination in the first case revealed hyperpigmented
plaque of 3 X 2 cm. with nodular surface, satellite lesions and
surrounding freckles (Fig 1). Single firm, mobile, tender left
inguinal lymph node measuring 1.5 X 1.5 cm was present.
 | Fig 1:
Multiple black nodular lesions associated with patch with uneven
pigmentation & irregular border Multiple hyperpigmented freckles |
|
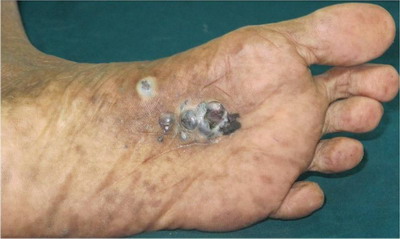
Second case revealed single, 4x2 cm, linear, hyperpigmented, plaque
with raised edge, irregular surface, overlying erosions and crusting
(Fig 2).
 | Fig
2: Single,
4x2 cm, linear, hyperpigmented, plaque with irregular surface,
erosions and crusting with raised edge |
|
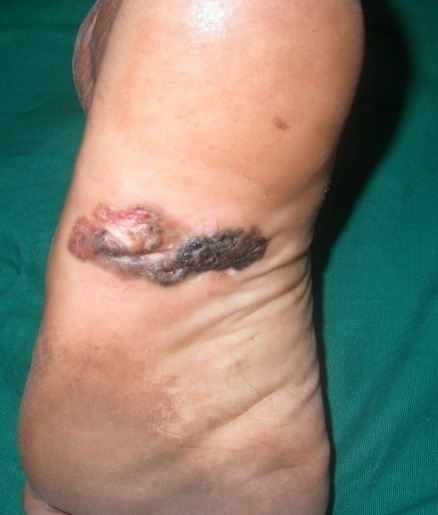
Examination of third case revealed single hyperpigmented
plaque 4x2 cm on periungual and subungual area with partial destruction
of nail plate and positive Hutchison's sign (Fig 3). Surprisingly
lymph node involvement was absent in both second and third case in spite
of longer duration of tumor.
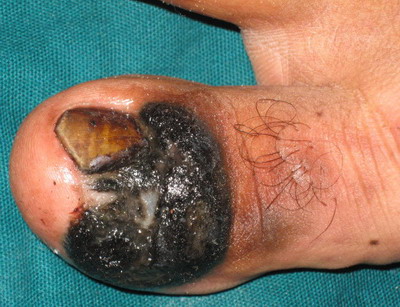 | Fig
3:
Lesion localized to right big toe in the form of black colored
plaque over periungual and subungual area with partial
destruction of the nail plate |
|
Laboratory and biochemical investigations of all the three patients
did not reveal any abnormality. Radiological investigations failed to
reveal metastasis to any organ in the body.
X- Ray of local parts did not show involvement of underlying bone.
Skin biopsy from the hyperpigmented nodule and plaques confirmed the
diagnosis of acral lentiginous melanoma. First case revealed compact
hyperkeratosis with diffuse infiltration of dermis with atypical rounded
and spindle shaped cells with focal pigmentary incontinence (Fig 4 &
5) while second case and third cases revealed lateral spread of
atypical melanocytes (Fig 6 & 7). Staging of melanoma is
mentioned in (Table 1).
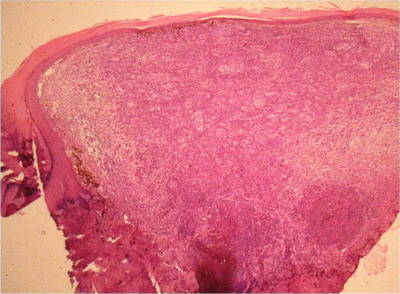 Fig
4: 100X, H & E: Case 1
Well Compact hyperkeratosis with diffuse infiltration of dermis
with atypical cells with focal melanin deposition. |
|
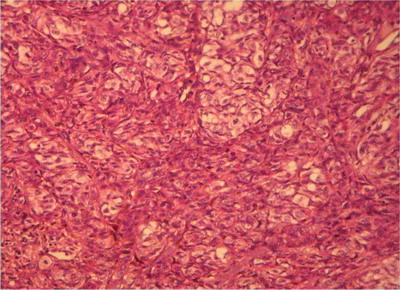 Fig
5: 400 X, H & E:
Case 1: Spindle shaped & rounded tumor cells seen with uniform
cytological atypia |
|
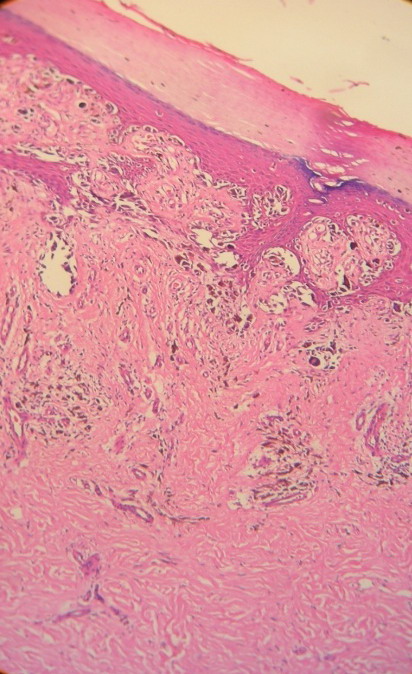 Fig
6: 100X, H& E:
Case 2: Lateral spread of atypical melanocytes along the basal
layers and in the dermis |
|
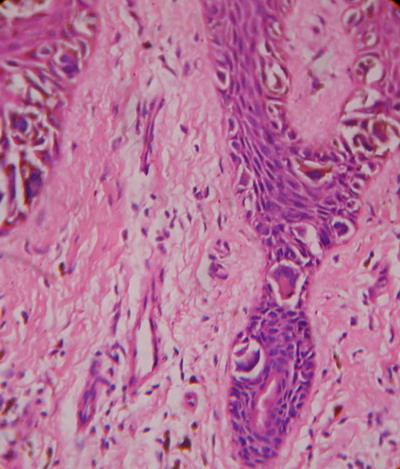 Fig
7: 400 X, H & E:
Case 2: Lateral spread of atypical melanocytes at the basal
layer. |
|
|
|
Case 1 |
Case2 |
Case3 |
|
Tumor thickness |
3 mm |
1 mm |
9 mm |
|
Overlying ulceration |
Absent |
Present |
Absent |
|
Lymph node metastasis |
Present |
Absent |
Absent |
|
Distant metastasis |
Absent |
Absent |
Absent |
|
AJCC staging |
T3aN2cM0 |
T1bN0M0 |
T4aN0M0 |
|
Stage III |
Stage IB |
Stage IIB |
|
Clark’s level |
IV |
II |
IV |
Table 1: Staging of melanoma
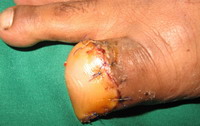
First patient underwent surgical excision with 1 cm margin and
inguinal lymph node block dissection (Fig 8, 9, & 10) while the
third patient underwent right great toe wide excision with
interphalangeal joint disarticulation and reconstruction with posterior
flap (Fig 11). Unfortunately second patient lost to follow up.
| |
| Fig 8: Case 1: Surgical
excision with 1 cm margin |
 | Fig
9: Case 1: Excised tumor with 1 cm margin |
|
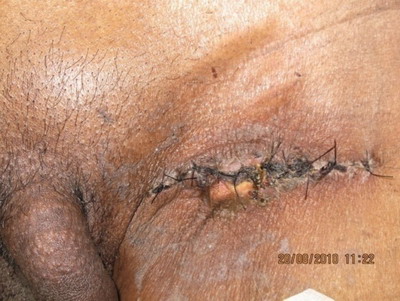 | Fig 10:
Case 1: Inguinal Lymph node dissection |
|
| |
| Fig 11: Case 3: right great
toe wide excision with interphalangeal joint disarticulation and
reconstruction with posterior flap |
Discussion
In 1976, Reed [1] first described ALM
as pigmented lesions on the extremities, particularly on plantar
regions, like the palms of the hands and soles of the feet, which are
characterized by a lentiginous (radial) growth phase evolving over
months or years to a dermal (vertical) invasive stage. It accounts for
about 2 to 3 % of all melanomas [2,3].
The overall incidence of cutaneous malignant melanoma (CMM) in darker
skinned individuals is low compared with whites; however, ALM makes up a
much higher proportion of CMM in darker skinned individuals (i.e.
blacks, Asians, and Hispanics).
Arrington et al [4] were the first to
note that this type of melanoma was the most common expression of
melanoma in blacks and those patients with ALM had a very poor
prognosis. In Reed's study, patients with ALM had a mean 3-year survival
rate of 11%. The poor survival rate of these patients may have been due
in part to delays in diagnosis [1].
Bradford [5] et al conducted a survey
through population based registry over a period of 20 years and found
distinct features of ALM compared to CMM. In their study overall
incidence of ALM was very low compared to other types of CMM i.e. 1.8
per 1000000 person-years without any sex predilection. The proportion of
ALM among all melanoma subtypes was greatest in people of dark races.
The mean age at diagnosis for ALM was 62.8 years, compared with 58.5
years for CMM overall. In their study lower extremity was most commonly
involved than upper extremity for ALM while for CMM trunk was the most
common site followed by upper and lower extremities. Comparing the tumor
thickness overall, CMMs were thinner than ALMs, with 70.0% of CMMs
diagnosed at 0.01 to 1.00 mm. In contrast, for ALMs, only 41.3% were
diagnosed at 0.01 to 1.00 mm, and 37.0% were diagnosed at thicker than
2.00mm. Tumor thickness was greater (2 mm) in men than Women with ALM.
Approximately 37.8% of ALMs were stage I, in contrast to 67.5% of CMMs.
Darker races showed 50 % of patients in the stage III than the white
races. Overall, patients with ALM had lower 5 and 10-year melanoma-
specific survival than for CMM. For ALM 10-year survival rates at 0.01
to 1.00 mm and 2.01 to 4.00mm were significantly lower than respective
CMM 10-year survival rates.
Clinical management of melanoma begins with an accurate diagnosis. A
suspicious lesion should be biopsied as the early diagnosis and
management also improves prognosis. A 1-3 mm margin of normal skin is
taken if the wound can be closed primarily. Wider margins should be
avoided to permit accurate subsequent lymphatic mapping. If removal of
the entire lesion creates too large a defect, then punch biopsy or
excision of a representative segment of the lesion is recommended. Once
a diagnosis of melanoma is made, the biopsy scar and any remains of the
lesion need to be removed to eradicate any remaining tumor. [6]
Histopathology of the ALM shows hyperkeratosis, marked acanthosis and
a proliferation of atypical melanocytes along the bases and sides of
rete ridges in a lentiginous pattern. The large atypical melanocytes
shoe large irregular nuclei, prominent nucleoli and the cytoplasm is
filled with melanin granules. In invasive tumors, the melanocytes in
dermis are spindle shaped and associated with sclerotic stroma. Nests of
atypical melanocytes can be seen at the junction or individual
melanocytes can be seen above basal layer up to stratum corneum.
The size of the surgical margins depends on the tumor thickness. For
in situ lesions a 0.5- to 1 cm margin of normal skin is adequate for
cure. Thin melanomas (≤1 mm) require a 1 cm margin to prevent local
recurrence; lesions between 1.01 and 2 mm should have a margin of 1-2
cm. For lesions between 2.01 and 4 mm, a 2 cm margin is recommended.
Extending the resection beyond 2 cm does not appear to decrease local
recurrence rates. Melanoma of fingers and toes requires digital
amputation. [7,8]
The surrounding tissue should be removed down to the superficial
fascia to remove all lymphatic channels. If the deep fascia is not
involved by the tumor, removing it does not affect recurrence or
survival rates. Generally, the wounds should be closed primarily. Larger
tissue defects may be closed with local rotational/advancement skin
flaps or a skin graft [9].
Regional lymph nodes metastasis is a poor prognostic sign. All
clinically positive lymph nodes should be removed by regional nodal
dissection unless unrespectable distant metastases are present.
Therapeutic lymph node dissection includes a superficial inguinal
lymphadenectomy. The deep (iliac and obturator) nodes should be removed
in the presence of clinical or radiographic evidence of deep node
involvement or if there are more than three positive superficial nodes
or when Cloquet's node is positive [10,11,12,13].
References
1. Reed RJ, New Concepts in Surgical Pathology of the
Skin, New York, NY: John Wiley & Sons, 1976:89-90.
2. Surveillance, Epidemiology, and End Results (SEER)
Program: SEER*Stat Database. National Cancer Institute, DCCPS,
Surveillance Research Program, Cancer Statistics Branch, Web site.
www.seer.cancer.gov. Accessed: July 2008.
3. Markovic SN, Erickson LA, Rao RD, et al. Melanoma
Study Group of the Mayo Clinic Cancer Center. Malignant melanoma in the
21st Century, part 1: epidemiology, risk factors, screening, prevention,
and diagnosis, Mayo Clin Proc, 2007, 82(3):364-380.
4. Arrington JH III, Reed RJ, Ichinose H, Krementz ET,
Plantar lentiginous melanoma: a distinctive variant of human cutaneous
malignant melanoma, Am J Surg Pathol, 1977,1(2):131-143.
5. Bradford PT, Goldstein AM, McMaster ML, Tucker MA,
Acral Lentiginous Melanoma Incidence and Survival Patterns in the United
States, 1986-2005 Arch Dermatol, 2009, 145(4):427-434.
6. Kosmidis C, Efthimiadis C, Anthimidis G, Grigoriou M,
Vasiliadou K, Ioannidou G, Makedou F, Baka S, Acral Lentiginous
Melanoma: A Case Control Study and Guidelines Update, Case Report Med,
2011;2011,670581. Epub 2011 Apr 6.
7. National Comprehensive Cancer Network Practice
Guidelines in Oncology. http://www.nccn.org.
8. Balch CM, Soong S-J, Smith T, et al, Long-term
results of a prospective surgical trial comparing 2cm vs. 4cm excision
margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol. 2001
Mar; 8(2):101-8.
9. Hansen S, Mathes S, Young D. Skin and subcutaneous
tissue, In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG,
Pollock RE, editors. Schwartz's Principles of Surgery, 8th edition, New
York, NY, USA: McGraw-Hill; 2005, pp. 1297-1315.
10. Coit DG, Extent of groin dissection for melanoma,
Surgical Clinics of North America, 1992, 1:271-280.
11. Coit DG, Brennan MF, Extent of lymph node
dissection in melanoma of the trunk or lower extremity, Arch Surg.
1989;124(2):162-166.
12. Shen P, Conforti AM, Essner R, Cochran AJ, Turner
RR, Morton DL. Is the node of Cloquet the sentinel node for the iliac/obturator
node group? Cancer J. 2000 Mar-Apr; 6(2):93-7.
13. Hughes TMD, A'Hern RP, Thomas JM, Prognosis and
surgical management of patients with palpable inguinal lymph node
metastases from melanoma. Br J Surg. 2000 Jul;87(7):892-901
© 2011 Egyptian Dermatology Online Journal
|