|
|
Abstract
Wound healing is a complex and dynamic process where the cell structures are restored to normalcy. The management of wound is currently by use of antiseptics and antibiotics which usually prevent / treat infection. But the intricate process of wound healing depends on cell migration and proliferation. Fibroblasts are the key cells responsible for initiating angiogenesis, epithelialization and collagen formation. Currently there is a dearth of scientific reports on agents that can facilitate the process of wound healing at cellular level. A polyherbal formulation consisting of extracts of Wrightia tinctoria, Aloe vera, Curcuma longa and Terminalia chebula was used to study the fibroblast cell migration and proliferation using scratch wound assay technique. The results of the study indicate that the polyherbal formulation may be useful in effective management of superficial wounds.
Keywords - Wound healing, Fibroblast, Scratch wound assay, Cell migration, Cell proliferation, herbal, Siddha
Background Wound or injury to skin can be due to intentional or accidental trauma. When the intact tissue is damaged, cells adjacent to it migrate to the site, proliferate, synthesize matrix components and tend to close the wound. Wound healing is a complex process of cellular and biochemical interactions involving various cells such as keratinocytes, fibroblasts and endothelial cells [1]. The wound healing process passes through four major phases such as hemostasis, inflammation, proliferation and remodeling [2]. The proliferative phase overlaps with the inflammatory phase. The most important cell is fibroblast which is responsible for initiating angiogenesis, epithelialization and collagen formation [3]. Use of antiseptics and antibiotics can only prevent or treat infections. Hence, preparations to augment other inevitable mechanisms responsible for wound healing are of prime importance and need to be explored. Several plant compounds are reported to have enhanced wound healing activity. The glycoprotein fraction from Aloe vera is reported to enhance cell proliferation and migration of keratinocytes thereby act as an effective wound healing agent [4]. The present study evaluates the wound healing effect of a Sidhha preparation (Test formulation) by 3T3 fibroblast cell migration and proliferation by scratch wound assay. A major advantage of this simple assay is that it mimics to some extent in vivo migration of cells. The Siddha preparation used in the present study contains the extracts of selective traditional plants with cell migratory, anti-inflammatory and anti-microbial properties viz. Wrightia tinctoria, Aloe vera, Curcuma longa and Terminalia chebula. This Siddha polyherbal formulation was made in a gel (carbomer) base for the study and is referred to as Thee Gel. Materials and Methods
Preparation of test formulation (Thee Gel): The coconut oil extracts of Wrightia tinctoria was prepared [5]. Propylene glycol extract of Aloe vera pulp, alcoholic extracts of Curcuma longa and Terminalia chebula were prepared separately and used. In brief, the extracts were incorporated into carbomer gel and bees wax base with the help of an emulsifier. The percentages incorporation of different extracts in test formulation are Wrightia tinctoria and Aloe vera at 0.4% and Curcuma longa and Terminalia chebula at 0.08%.
Cell culture Mouse fibroblast 3T3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 2 mM glutamine and 100μg/ml each of penicillin and streptomycin and incubated at 5% CO2 at 37°C [1].
Wound healing-migration assay Cells were grown to confluence on a 24-well dish, the medium was aspirated, and serum starved for 24 hours. Fresh medium with or without Test formulation was added. A single stripe (500
μm wide) was scraped on the cell-coated surface with a disposable plastic pipette tip and the wound was allowed to heal for 24 hours. The average extent of wound closure was evaluated by measuring the width of the wound. The speed of migrating cells into the wound area was examined and photographed [6,7]. Cell migration was assessed by microphotography. The fibroblasts were treated with varying concentrations of test formulation and were examined at 12 and 24 hours after treatment. Untreated control, 10nM epidermal growth factor (EGF) treated set for positive reference and placebo treated set were also maintained. Fibroblasts were treated with 5, 10, 25 and 50
μg/ml of Test formulation, incubated for 24 hours. The extent of cell migration was photographed and measured using image analyzing software. Each experiment was performed in triplicate.
Cell proliferation assay Fibroblast 3T3 (103) cells were seeded in a 96-well culture plate in a humidified 5% CO2 atmosphere. Cells were serum starved for 24 hours and then incubated with 5, 10, 25 and 50μg/ml of Test formulation and further incubated for 24 hours. After 24 hours 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was added and formazan product was dissolved in 100μl of DMSO. Absorbance at 570 nm was measured with microtiter plate reader and cell viability was determined by tryphan blue exclusion method. Results
Cell migration assay Test formulation treatment enhanced the cell migration of 3T3 fibroblasts when tested by scratch wound assay. After 12hr of treatment with 50μg of Test formulation, the migratory nature of the fibroblast could be seen by microscopic examination. Complete covering of the wound was observed within 24 hours of treatment, similar to that of EGF. This response was not recorded either in the cell culture plate maintained as untreated control or placebo treatment
(Figure 1).
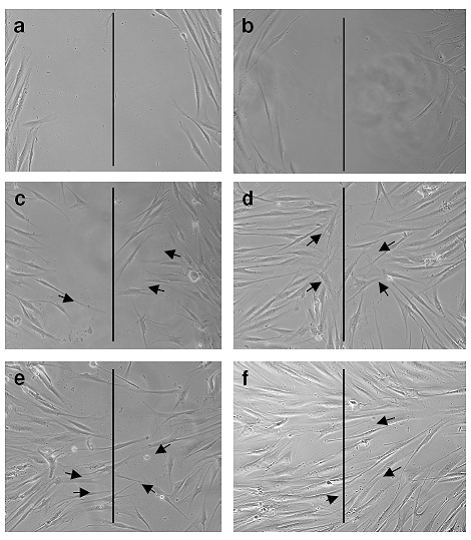 Fig 1:
Cell Migration Assay
a. Control-12 hours
b. Control-24 hours
c. Test formulation-12 hours
d. Test formulation-24 hours
e. EFG-12 hours
f. EFG-24hours
Cell migration assay shows the movement of fibroblasts (indicated by
arrow lines towards the imaginary line drawn in the center of the
image) captured at 12 and 24 h after incubation using phase-contrast
microscope.
|
|
Fibroblast proliferation assay An increased proliferation of fibroblast in response to treatment with test formulation was observed. The rate of proliferation was directly proportional to the concentration. However, concentration above 50μg/ml did not greatly influence the proliferation rate. 30% increase in the proliferation of fibroblast in response to test formulation at a concentration of 50μg/ml was demonstrated, whereas in the positive control, EGF showed 70% increase in cell proliferation. Cell proliferation was insignificant in untreated and placebo treated control
(Figure 2).
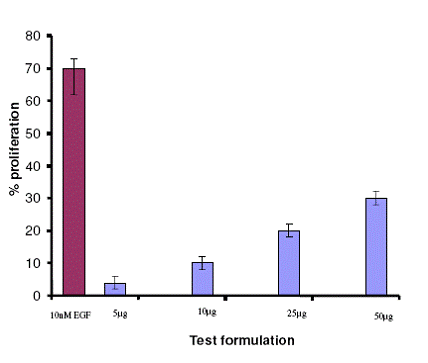 | Fig
2: Effect of test formulation on cell proliferation |
|
Discussion
The present study clearly suggests that test formulation enhances the cell proliferation and migration of 3T3 fibroblasts. Cell proliferation and cell migration are two important events necessary for wound healing [8]. We have chosen 3T3 fibroblast cell line because, the fibroblasts play a major role in wound healing process, besides keratinocytes. Test formulation contains the oil extract of Wrightia tinctoria which is known to have several medicinal properties including anti-inflammatory activity [9]. The glycoprotein of Aloe vera has wound healing effect as evidenced by enhanced cell proliferation and migration [3]. The antimicrobial and wound healing effect of Curcuma longa and Terminalia chebula are well-documented [10-14]. The alcoholic extract of Terminalia chebula increases the protein, DNA and collagen synthesis in the granulation tissue during wound healing [14]. However, the poly herbal extract (test formulation) of Wrightia tinctoria, Aloe vera, Terminalia chebula and Curcuma longa and its wound healing effect as evidenced by the enhanced cell proliferation and cell migration of fibroblast in a suitable vehicle base (gel) is novel and has not been reported earlier. The polyherbal extract was incorporated into a carbomer gel and bees wax base. The gel has an outer water phase, which on application cools the wound surface and enables the extracts to permeate through the skin. The inner phase of the gel consisting of bees wax forms a coating on the surface of the skin to ensure the sustained availability of the herbal actives and prevent water loss. Although the test formulation could not be tested on all four stages of wound healing process, the findings of the present study gives suggestive evidence for the possible usefulness of this topical herbal gel preparation in the management of superficial wounds and first degree burns. Conclusion
The combination of extracts of Wrightia tinctoria, Aloe vera, Terminalia chebula and Curcuma longa has a definite effect in promoting migration and proliferation of fibroblasts. Moreover, the novel delivery system (carbomer based gel with bees wax) is an encouraging formulation mode for the delivery of the polyherbal actives in the management of superficial wounds and first degree burns. References
1. H Tao, J Anthony, Berno, RC David, AF Kelly: In vitro human keratinocyte migration rate are associated with SNPs in the KRT1 interval. PLoS ONE 2007, 2(8): e697
2. Krishnan P. The scientific study of herbal wound healing therapies: Current state of play (2006) Current Anaesthesia and Critical Care, 17 (1-2), pp. 21-27.
3. A Komarcevic: The modern approach to wound treatment. Med Pregl. Jul- Aug 2000, 53(7-8):363-8.
4. J Gallagher, M Gray: Is Aloe vera effective for healing chronic wounds?. Journal of Wound Ostomy continence Nurs 2003, 30(2): 68-71.
5. Clinical and Experimental studies on the efficacy of 777 oil, a siddha preparation in the treatment of Kalanja padai (Psoriasis). Research Monograph on 777 oil. Published by Central Council for Research in Siddha and Ayurveda, Ministry of Health and Family Welfare, Govt. of India, New Delhi. 1987.
6. SP Denker, DL Barber: Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. Journal of Cell Biology 2008, 159:1087-1096.
7. F Entschladen, TL Drell, K Lank, K Masur, D Palm, P Bastian, B Niggemann, KS Zaenker: Analysis of methods of human cell migration. Experimental Cell Research 2005, 307(2): 418-426.
8. Chun-Chi Liang, Ann Y Park, Jun-Lin Guan: In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols 2007, 2(2) 329-333. Published online 1 March 2007; doi:10.1038/nprot.2007.30.
9. P Bigoniya, A Shukla, GP Agrawal, AC Rana: Pharmacological screening of Wrightia tinctoria bark hydro alcoholic extract. Asian Journal of Experimental Science 2008, 22(3): 235-244.
10. GS Sindhu, AK Singh, D Tharoor, KK Banaudha, GK Patnaik, RC Srimal, KM Radha: Enhancement of wound healing by Curcumin in animals. Wound Repair & Regeneration 1998, 6(2): 167-177.
11. N. Niamsa and C. Sittiwet,. Antimicrobial Activity of Curcuma longa Aqueous Extract. Journal of Pharmacology and Toxicology, 2009. 4: 173-177.
12. KJ Kim, HH Yu, DD Cha, SJ Seo, NY Choi, YO You: Antimicrobial activity of Curcuma longa against methicillin - resistant Staphylococcus aureus. Phytotherapy Research 2005: 19(7): 599-604. PMID: 16161063 [Pubmed - MEDLINE]
13. L Suguna, S Singh, P Sivakumar, S Padmavathi, G Chandrakasan (). Influence of Terminalia chebula on dermal wound healing in rats. Phytotherapy Research 2002, 16(3): 227-231.
14. Kannan, P., S.R. Ramadevi, H. Waheeta, 2009. Antibacterial activity of Terminalia chebula fruit extract.African Journal of Microbiology Research, 3(4): 180-184.© 2012 Egyptian Dermatology Online Journal |


