|
|
Abstract
Background: Stable vitiligo and lesions with depigmented hairs, indicating the depletion of the melanocyte reservoir in the hair follicle, mostly fail to repigment by conservative therapies. In these cases, surgical techniques may be indicated but are often time-consuming and lead to undesired effects.
Objectives:
Evaluation of the transplantation of non-cultured melanocytes by two methods versus dermabrasion and thin split thickness skin grafting in cases of non progressive leukoderma.
Patients:
30 patients with non progressive leukoderma. Methods:
- Dermabrasion and thin split thickness skin grafting in 10 patients
- Autologous grafting with non cultured melanocyte suspension and this suspension was injected into liquid nitrogen induced blisters in 10 patients and was applied to the dermabraded depigmented skin in another 10 patients.
Results: The first group: 40% of the patients showed good results, 20% excellent results, 20% fair results and 20% poor results. The second and third groups: in each group 7 out of 10 (70 %) patients showed excellent to good results and in 3 patients results were poor.
Conclusion: Transplantation of melanocytes suspension gave a better result and rapid response
Keywords: Stable vitiligo, leukoderma, skin graft, melanocytes transplantation, autologous. Introduction
Vitiligo is an acquired depigmentary disorder of great cosmetic importance affecting approximately 0.5% to 1% of the world population. [1] It is characterized by the total or partial loss of melanocytes from the epidermis. [2] It is a major psychosocial problem, especially in people with dark skin. Many treatment modalities are currently used for vitiligo, such as psoralen plus ultraviolet A (PUVA), narrowband ultraviolet B (NB-UVB), excimer lasers, topical steroids, topical immunomodulators, and calcipotriol. [3,4] In patients with stable leukoderma (e.g., segmental vitiligo), surgical methods can be alternative therapeutic options.[5,6] These surgical techniques are based on a common principle: to transplant autologous melanocytes from a normal pigmented donor skin to depigmented area. Many surgical techniques for repigmenting vitiligo have been devised over the years and can be broadly divided into tissue and cellular grafting. Tissue grafts include full-thickness punch grafts, thin dermoepidermal grafts, and suction epidermal grafting. With these tissue grafts, only a limited surface area can be treated per treatment session. [5,6] Cellular grafts include cultured pure melanocytes suspension and non-cultured epidermal cellular suspensions (mixture of melanocytes and keratinocytes) [7-15] these epidermal cells can also be co-cultured to epithelial sheet grafts. The major advantage of these suspension and culturing techniques is that, they permit treatment of affected skin manifold larger than the donor area. However, culturing techniques are time consuming, expensive due to the culturing time of several weeks, and require highly trained personnel and well-equipped tissue laboratories. Furthermore, the use of specific growth factors and additives in the culture medium (e.g.12-O-tetradecanoyl-phorbol 13-acetate/TPA), pose safety concerns. [16-20].
Aim of the work 1- Evaluation of the transplantation of non-cultured melanocytes versus dermabrasion and thin split thickness skin grafting in cases of non progressive leukoderma. 2- Comparison between both techniques as regards:
- Repigmentation and its extent.
- The rate of success as regards:
- The type of vitiligo (localized or generalized).
- The site of the lesion.
- The effect of additive post operative treatment using topical PUVA.
- The feasibility of either technique considering expenses, healing time, complications and cosmetic appearance.
Patients: This study included 30 patients with non progressive vitiligo or stable forms of leukoderma (post traumatic, post burn and piebaldism). The selected candidates for this study were patients attending Alexandria University Main Hospital or those referred from other parts of the country.
Inclusion criteria Patients with stationary disease with no new lesions or increase in the size of the existing lesions for at least 2 years, in whom these lesions were not responding to medical or UV treatment.
Exclusion criteria 1- Cases of active disease: and the activity was determined by the presence of at least one of the following criteria:
- Freshly erupted lesion.
- Lesions increasing both in width and longitudinal diameter.
- Grouping of small lesions together.
- Appearance of new lesions away from the primary one.
2- Patients with vitiligo covering more than 70% body surface area, as the level of anti-melanocyte antibodies were reported to correlate with the extent as well as the activity of vitiligo. 3- Patients with associated autoimmune diseases especially autoimmune thyroiditis, diabetes mellitus or pernicious anemia (depending on the history).
Method Every patient in this study was subjected to the following:
- History
- Physical examination
- Explanation of the procedure
- Baseline investigations included complete hemogram and coagulogram, HIV, and Hepatitis B and C virus serology.
- Photography
Informed consent was taken from all patients participating in the study, The study was approved by the Alexandria Faculty of Medicine, Research Ethics Committee. The 30 patients of the study were subdivided into 3 equal groups:
The first group (n = 10) was subjected to dermabrasion and thin split thickness skin grafting according to Najoo et al 1998[21] by the following method. Under general anesthesia, the donor and the recipient areas were cleaned and sterilized with povidone-iodine, followed by 70% alcohol. The recipient (vitiliginous) area was dermabraded with a high speed dermabrador (Osada XL-30W Electric Handpiece System, Dermatologic Lab & Supply, Inc. 608 13th Ave. Council Bluffs, IA USA 51501-6401) and normally fitted with a regular diamond fraise until uniform pin-point capillary bleeding was seen
(Fig. 1). Denuded areas were covered with gauze moistened with Saline solution pending transplantation.
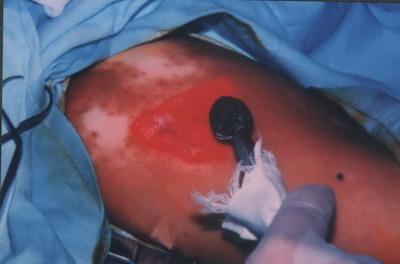 | Fig 1: Dermabrasion
of the depigmented area with a high speed
dermabrador. |
|
From the donor (pigmented) area (usually the inner aspect of the thigh), a very thin split thickness skin graft (0.1-0.15 mm thick) was removed with skin graft knife that is adjusted with 0.06 shield at the level control for obtaining the required thin graft
(Fig. 2)
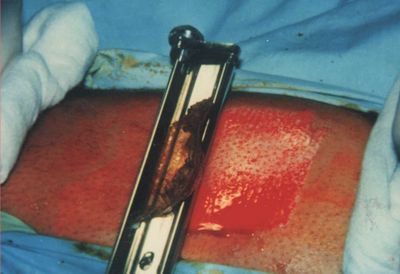 | Fig
2: obtaining the split skin graft with the skin graft knife from the
medial aspect of the thigh. |
|
The size of the graft was justified according to the size of the depigmented area. After removal the thin split thickness skin graft, it was moistened with sterile saline solution, to insure that the graft did not become dry before being applied . The slightly bleeding doner area was covered with sofratulle ( lanolin gauze dressing containing 1% framycetin sulfate) or Kaltostat sheet (an absorbent fibrous fleece composed of the sodium and calcium salts of alginic acid in the ratio of 80:20.) and covered with adhesive bandage
(Fig .3).
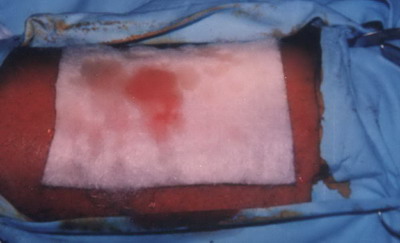 | Fig
3: Covering the donor area with the Kaltostat sheet after obtaining
the split skin graft. |
|
The graft was gently placed on the dermabraded a chromic area, where the surface was flattened and stretched over the dermabraded area. Some stitches (at the borders) were taken to fix the graft to the recipient area and then covered with saline moistened gauze & sometimes tied over to fix the graft by dry sterile gauze and adhesive bandage. The patient rested in a hospital bed for at least 4 hours after the procedure had been completed. The bandages were removed after 1-2 weeks .In the following weeks the patients were encouraged to expose themselves moderately to the sun in a slowly increasing doses. The patients were followed up every 2 weeks for one and half year. The second group (n = 10) was subjected to autologous grafting with non cultured melanocyte suspension. The suspension was formed of epidermal cells mainly keratinocytes and melanocytes and this suspension was injected into liquid nitrogen induced blisters. According to Gauthier and Surleve -Bazeille in 1992 [12] The treatment is carried out in two steps on two consecutive days:
The first day: A- The donor (pigmented area): Under general anesthesia, a superficial skin samples were obtained with skin graft knife from the medial aspect of the thigh after shaving and sterilization with povidone-iodine. The skin samples were immediately immersed in 0.25% trypsin solution in a test tube and incubated for 18 hours at 40C (in CO2 incubator)
B- The recipient (depigmented) area:
In the depigmented area, blisters were induced by the application of liquid nitrogen with a cotton tipped applicator for 20 to 25 seconds in several areas separated by a distance between 1 &2 cm
(Fig.4)
 | Fig
4: Liquid nitrogen induced blisters in the depigmented area ( 0.1 ml
of melanocytes suspension was injected in each blister ). |
|
The second day :
A- Preparation of the melanocytes suspension : Under complete aseptic condition (under laminar air flow)
(Fig. 5).The epidermis was separated from the dermis with fine forceps. The epidermal samples were placed for 15 minutes at room temperature in a solution of EDTA and then placed in saline solution (1ml of solution was used for each 1cm2 of skin).
 | Fig
5: Preparation of the melanocyte suspension under the laminar air
flow. |
|
The exposed basal layer of the epidermal sheet was rubbed gently with spatula to separate the epidermal cells (keratinocytes and melanocytes). The solution and the fragments were then agitated to detach the cells. The remnants of the skin fragments were removed & the cellular suspension was aspirated in an insulin syringe with a 25 gauge needle.
B- Injection of the melanocytes suspension : About 0.1ml of the suspension was injected in each blister. Before injection, the viscous blister fluid was partially aspirated. After the injection, the patient remains at rest 20 minutes to allow sedimentation of the cellular suspension. The grafted areas were then bandaged where the tops of the blisters served to hold the transplanted cells in place. The bandages were removed after one week and the patients were followed up every 2 weeks for one & half year. The third group (n = 10) was treated by transplantation of a basal cell layer enriched suspension devoid of stratum corneum and stratum granulosum that was applied to the dermabraded depigmented skin by the following method described by Olsson and Juhlin in 1998 [22]
Donor site :( normal pigmented site): Under general anesthesia, a superficial skin samples were obtained with skin graft knife (fitted with a 0.06 shield) from the medial aspect of the thigh (1/10 -1/4 of the size of the recipient area) after shaving and sterilization with povidone-iodine. After the skin samples were obtained, the donor site was covered with sterilized gauze or Kaltostat sheet which was left on for 7 days.
Preparation of the sample: The thin skin sample was immediately put in a test tube containing S- MEM spinner [22] and was transferred in the laboratory (under the laminar air flow) to a Petri-dish 6 cm in diameter where it was washed with Joklik,s modified minimum essential medium and refurnished with 5 ml of trypsin / EDTA solution. The sample was turned back and forth to insure that it came into complete contact with the solution. Finally with the epidermis side upwards, it was torn into pieces of 2cm & the Petri dish was incubated at 37oC for about 50 minutes After incubation the trypsin /EDTA solution was removed and about 2ml trypsin inhibitor was added to the dish to discontinue the trypsin action. The epidermis was removed from the dermis with a fine forceps and the dermal pieces were then discarded and the epidermal pieces were processed to smaller fragments and transferred together with the trypsin inhibitor to the test tube that was vortex mixed for 54 seconds and then centrifuged for 6 min at /50 g. the supernatant was removed and the pellet was centrifuged once again, and then resuspended in a total volume of 0.8 ml M2 medium. The suspended pellets were transferred to 1 ml/ syringe in steps to avoid any cell loss. The suspension thus contained cells from the basal cell layer and about half of the stratum spinosum.
Recipient site (The depigmented site): The recipient areas were cleaned with alcohol, and local anesthesia using 1% xylocaine was injected in these areas, few minutes before the procedure. Superficial dermabrasion on the recipient site by a high speed dermabrador fitted with a diamond head to remove the epidermis
(Fig.6) Denuded areas were covered with gauze moistened with phosphate buffered saline before transplantation. The suspended cells were applied with a syringe and spread out with its tip. The cells were covered with vaseline gauze, M2 moistened gauze and finally bandaged. The patient rested in bed for 4- 5 hours after the procedure. The bandage were worn for a week and then carefully removed.
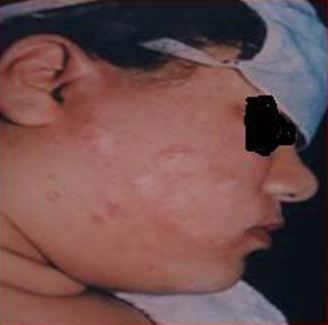 | Fig
6: Superficial dermabrasion of the depigmented area . |
|
Post operative measures
- In all the 3 groups, the patients received broad spectrum antibiotics (ampicillin or erythromycin) for at least 7 days after the operation (to guard against infection).
- Patients were followed every two weeks for the first 3 months then every month for one and half year. After about 2 weeks from the operation, the patients were encouraged to expose themselves moderately to the sun in slowly increasing doses. In patients with slow repigmentation response a topical PUVA (0.005% psoralen which was applied 15 minutes prior to UVA irradiation). The initial radiation dose was 0.5 J/cm2. The dose was increased in 20% increments until noticeable erythema developed then the treatment was continued at that dose for 3 months after the operation (once weekly) to accelerate the distribution of pigment. The devise used was UVA fluorescent tubes cabin (Philips TL 100W/10R, Philips Eindhoven, the Netherlands) with emission spectrum between 315 and 400 nm and a maximum wave length of 355 nm.
Results
Group 1: In 8 out of 10 (80%) patients, the usual course of repigmentation was as follows: one week after the operation there was necrosis, darkness and dryness of the border of the graft
(Fig.7) .At 1 to 2 weeks later, a loss of the necrotic margin of the graft was noticed , then slight contraction of the graft with the appearance of depigmented zone around the graft which was observed 3-4 weeks after the operation
(Fig. 8) This was improved after about 6 months on repeated sun exposure or topical PUVA
(Fig . 9) .
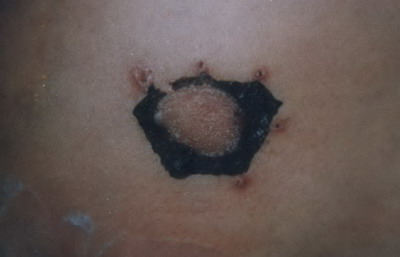 | Fig
7: Necrosis, darkness and dryness of the border of the graft one
week after the operation. |
|
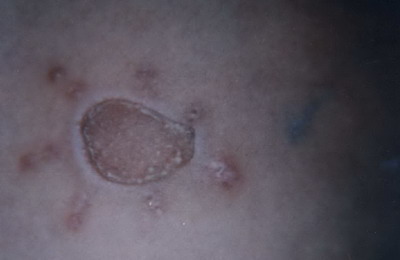 | Fig
8: The necrotic margin of the graft was lost with slight contraction
of the graft and appearance of depigmented zone around the graft 3-4
weeks after the operation. |
|
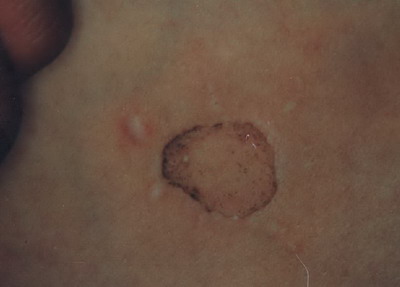 | Fig
9: Repigmentation of the borders after about 6 months on repeated
sun exposure & topical PUVA. |
|
In 4 out of 10 (40%) of the treated patients, there was a transient elevated border of the graft in the first 3 months after treatment
(Fig.10), in 2 (20 %) cases loss of the graft and keloid formation in 1 case. As regard the objective assessment: 2 patients (20%) showed excellent results
(Fig 11 a, b), 4 (40%) patients showed good results
(Fig 12 a, b), 2 (20%) patients showed fair results
(Fig 13 a, b) and 2 (20%) showed poor results
(Fig 14 a, b).
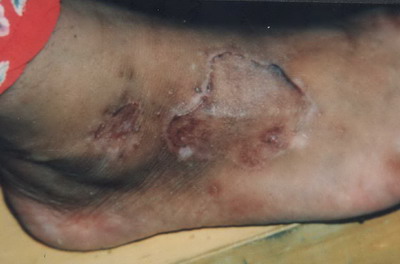 | Fig
10: Transient elevated borders of the graft in the first 3 months
after the operation . |
|
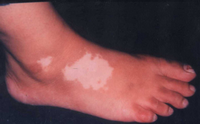 | Fig 11a:
The patient before treatment |
|
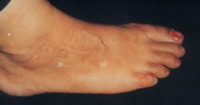 | Fig 11b: Excellent
result after treatment with split skin graft. |
|
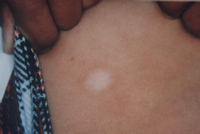 | Fig 12a:
The patient before treatment |
|
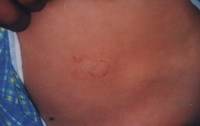 | Fig 12b:
Good result after treatment with split skin graft. |
|
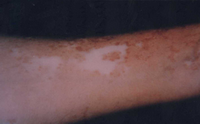 | Fig 13a:
The patient before treatment |
|
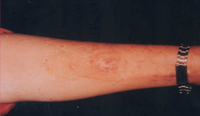 | Fig 13b:
Fair result after treatment with split skin graft. |
|
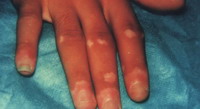 | Fig 14a:
The patient before treatment |
|
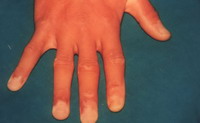 | Fig 14b:
Poor result after treatment with split skin graft. |
|
Group 2: Repigmentation started 3 to 4 weeks as bullae were dried and the pigmentation appeared in stippling form that increased in size during the observation period
(Fig.15 a, b) . Pigment spread rapidly in the first 3 months and then slowed down.
 | Fig 15a:
Multiple bullae induced at the depigmented area 24 hours after
application of liquid nitrogen |
|
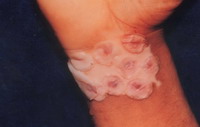 | Fig 15b:
Dryness of the bullae 2-3 weeks after the operation with the
appearance of pigment. |
|
Objective assessment: 7 out of 10 (70 %) patients showed excellent to good results
(Fig .16 a, b and Fig.17 a, b). In 2 (20%) cases in this group the failure of induction of bullae in the recipient site was considered as a method side effect.
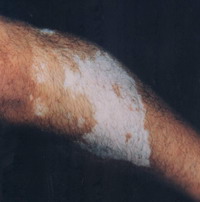 | Fig 16a: The patient before treatment |
|
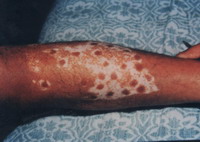 | Fig 16b:
Excellent result after treatment
with melanocytes transplantation on
bullae induced with liquid nitrogen. |
|
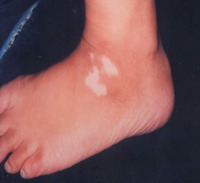 | Fig 17a: The patient before treatment |
|
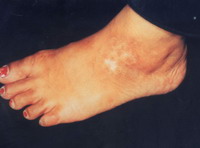 | Fig 17b:
Good result after treatment with
melanocytes transplantation on
bullae induced with liquid nitrogen. |
|
Group 3: Repigmentation occurred in 9 out of 10 (90 %) cases. In the first 2 weeks after the operation there was erythema, scabs and crustation at the site of dermabrasion then one week late, the pigment started to appear that increased in size during the observation period.
Objectively: 7 (70 %) cases showed excellent to good results
(Fig .18 a, b and Fig .19 a, b) and the method side effect in this group was slight hyperpigmentation at the recipient site in 3 (30%) cases
(Fig. 20).
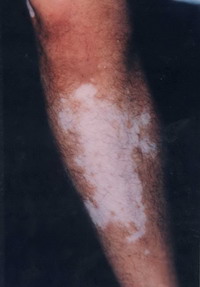 | Fig 18a:
The patient before treatment |
|
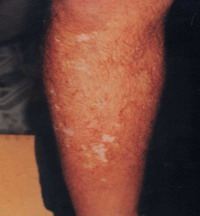 | Fig 18b:
Excellent result after treatment
with melanocytes transplantation
on dermabraded skin. |
|
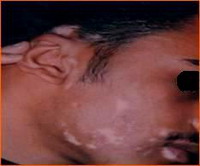 | Fig 19a:
The patient before treatment |
|
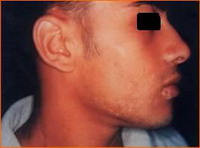 | Fig 19b:
Excellent result after treatment
with melanocytes transplantation
on dermabraded skin. |
|
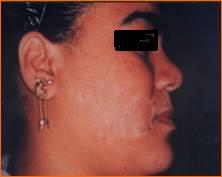 | Fig
20: slight hyperpigmentation at the
site of melanocytes transplantation
on dermabraded skin. |
|
Discussion
The management of vitiligo is still a challenge for dermatologists because a portion of patients fail to respond to the currently available treatment modalities. Surgical therapeutic modalities have a major role to play in lesions of non progressive vitiligo and stable forms of leukoderma (halo nevi, nevus depigmentosus, chemical leukoderma, post burn leukoderma, piebaldism). [23] This study included 30 patients with non progressive vitiligo and stable forms of leukoderma (post traumatic, post burn and piebaldism). The 30 patients studied were subdivided into 3 equal groups: The first group (n = 10) were subjected to dermabrasion and thin split thickness skin grafting (SSG).The procedure was not difficult to perform, however dermabrasion and harvesting a thin SSG with the skin graft knife requires good training and experience. The success of SSG depends on obtaining a very thin graft and proper immobilization of the treated area at least for the first three days, as there are three biological changes which follow skin grafting. [24] Graft take adherence: There are two phases in this stage. In the first 72 hours of the placement of the graft adherence of the graft is due to fibrin bonding and the graft appears pink. Hence, these first 72 hours are most crucial for graft uptake; bleeding, infection, and mechanical movement due to improper immobilization of the area can lead to graft failure at this stage. Hence strict immobilization in the first three days is very essential. The second phase begins with the onset of vascular anastomosis and fibrovascular growth; graft revascularization: In this stage there is a connection between the graft and host vessels, with the formation of new vascular channels. Insufficient vascular proliferation, development of a thick layer of fibrin or hematoma or seroma can lead to failure of graft uptake in this stage. Hence compression is useful.
Contracture: There is contracture of the graft when it is harvested because of the contraction of the elastin fibers. Contracture also occurs at the recipient site. These two factors lead to achromic fissures and perigraft halo. Overlapping of graft edges at the recipient site can prevent these complications. The mechanism of repigmentation in this procedure is by covering the leukodermic area by an equal sized pigmented SSG after dermabrasion of this area. It is possible that growth factors and cytokines released during wound healing help in migration, transplantation, and multiplication of melanocytes. [25] The most important factor that affect the response to SSG is the site of vitiligo. In this study it was found that vitiligo of the fingers and vitiligo on the neck are not suitable for treatment by this method due to mobility of these areas which leads to loss of the graft. In our study SSG in one case (skin type IV) with leukoderma on the chest the site of stitches at the periphery of the graft showed keloid formation. A firm conclusion cannot be deduced from a single case, however it will be safe to avoid keloid prone areas. In addition, in our study it was found that; cosmetically; the face is not a suitable site for treatment by SSG that had to be fixed by stitches at the periphery for fixation. Also SSG takes more than one year to become cosmetically acceptable and matched with surrounding skin. This finding was in contrast to the study done by Farah Sameem et al 2011 [25] in which The donor graft was taken by means of Humby's or Slivers knife. After needling the graft, it was placed on the dermabraded bed and fixed by surgical glue. A composite dressing was placed at both site for repigmentation of vitiligo on the face with good to excellent results. Whether the thinness of the SSG graft in his study in contrast to thicker SSG or the fixation of the graft by surgical glue instead of stitches in our study are the causes of the difference noticed, this facts need further elucidation . Furthermore, too thin SSG do not have enough melanocytes to produce pigmentation and skin that is too thick cannot be used in areas with thin skin. [21,26] In addition Farah et al stated that the thickness of the graft was a defining factor for the satisfactory response. Thinner grafts that were shed off completely after donating the melanocytes to vitiliginous areas were the best ones. Thicker grafts would result in a stuck on appearance at recipient site and infection and scarring at donor site. [25] In our study limited area can be treated by SSG in contrast to the study done by Srinivas 2004 [27] in which he used meshed SSG to cover larger depigmented areas. In this study topical PUVA helps in repigmentation of the halo rim around the graft but in the study done by AL-Mutairi 2010 [28] better results were obtained after excimer laser. . The second group: was treated with transplantation of non cultured melanocytes suspension in liquid nitrogen induced bullae in the depigmented area. In this method the epidermal cell suspension formed of keratinocytes and melanocytes. It seems important not to separate keratinocytes from melanocytes because factors furnished by keratinocytes sustain the melanocytes growth. [12] It was assumed that, the growth of melanocytes administrated as a suspension is determined by stimulatory and inhibitory factors and that in each patient, factors having the upper hand, determine the outcome of the response. Among the stimulatory factors are: the growth factors released by keratinocytes, whether present in the suspension or from the surrounding recipient area and these growth factors act as migratory and melanogenic factors for melanocytes. [29] On the other hand, factors hindering the growth and multiplication of melanocytes are: the immune response (which was not present in stable vitiligo) [30], subclinical infection and the presence of fluid inside the bullae that may contain antibodies and immunocytes besides its being a physical obstacle. In addition, the site of the recipient area may play a role in the proper take of pigment either due to inadvertent trauma, mobility or vascularity. The association of lower age with good response might be explained by the finding that, melanocytes of patients older than 40 years have a lower potential to proliferate and produce pigment in culture. A similar behavior could also be present in vivo [31]. However Gauthier and Surleve - Bazille 1992, found no influence of age of the patients on the outcome of therapy. [12] In our study, it was found that the darker the skin the better was the repigmentation and may be explained the fact that the melanocytes in dark skinned person are hyperactive with fully melanized melanosomes. In this study the appearance of pigment at the sites of melanocytes injection took about 3 to 4 weeks and this interval is the same as that mentioned by Gauthier and Surleve - Bazille 1992 [12]. The long time taken for repigmentation was explained by that there was a time taken for the melanocytes to settle down, then to proliferate to an appreciable number, then to migrate and produce melanin. Another speculated cause for the delay of repigmentation in transplantation of melanocytes by this method is that the fluid present inside the bullae could have hindered proper growth of the cells. In the third group , 10 patients were treated by transplantation of a basal cell layer enriched suspension devoid of stratum corneum and stratum granulosum that was applied to the dermabraded depigmented skin according to Olsson and Juhlin in 1998. [22] This method is a laboratory method and needs many reagents and also time consuming but it gives better results especially on the face, neck and trunk , a similar results were also found in many studies [32-39], however a poor results were found on the acral parts Mulekar et al 2009 [39], reported recently good results with epidermal cellular grafting on "more difficult-to-treat sites" (fingers, toes, elbows, …), but they mentioned that multiple sessions were often necessary.[40] The best results obtained in this study by this method occurred in patients with segmental vitiligo (5 cases) with no loss of pigment during the follow up period. Similar results were found by VanGeel 2010 [14]. As segmental vitiligo stabilizes spontaneously, in general, within the first year of onset, this remains the best surgical treatment indication.[14]. In this study 3 patients with stable generalized vitiligo were treated in some areas by this method with a good results but 2 of them reported a loss of pigment after 3 years of follow up, so a minority of patients with generalized vitiligo are suitable for a surgical intervention, as this type of vitiligo in general extends over time and the intervention does not alter the underlying pathoetiology. The adverse effects on recipient and donor areas are in general minimal or absent but in one patient with segmental vitiligo there was a hyperpigmentation at the recipient area. The same observation was found according to the available literature. [14] This color mismatch was not disturbing according to the majority of patients. [14] At the donor site minor textural skin changes were observed in this study. The majority of these patients in this study accepted this side effect as this was hardly visible by clinical inspection. Hyperpigmentation at the donor site has been reported by several authors [14].
Conclusions
1- Surgical treatment of vitiligo is suitable for patients who tried medical therapy for a very long time without any benefit and their disease is stationary and involves less than 20 % of his body . 2- The split thickness skin graft (SSG) is a rapid method for treating non progressive vitiligo or stable forms of leukoderma, but it needs a well trained surgeon to obtain an appropriate thickness of SSG. 3- SSG is not suitable for highly mobile areas or keloids prone areas and not suitable for the face. 4- SSG was suitable for any age and any skin type 5- Transplantation of non cultured melanocytes suspension in liquid nitrogen induced blisters is a laboratory procedure, which we adopted because we thought that it might give better results than SSG. It is more tedious and more time consuming. 6- Transplantation of basal cell layer enriched cell suspension on dermabraded depigmented skin also is a laboratory procedure that needs a lot of chemical substances that encourage the growth of melanocytes. It is more expensive than the previous two methods but it gives a better results and rapid response. 7- The above mentioned 3 methods are not suitable for acral vitiligo (vitiligo on the fingertips) as these areas are resistant . 8- Split thickness skin graft and melanocytes transplantation were alternative treatment for localized forms of vitiligo if currently used medical treatment failed. 9- As compared to noncultured melanocyte suspensions, no reagents, laboratory facilities or expensive elaborate equipment are required for SSG. 10- Transplantation of non cultured melanocytes suspension can be used to treat large areas than that treated by dermabrasion and split thickness skin graft. 11- Patients with Koebner's phenomenon must be excluded, for fear of appearance of vitiligo at the site of surgery. 12- It is recommended that, further research studies must be done on larger number of vitiligo patients and for longer follow up period for more evaluation of the effect of both modalities. References
1. Taieb A, Picardo M. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res 2007;20:27-35.
2. Westerhof W, d'Ischia M. Vitiligo puzzle: the pieces fall in place. Pigment Cell Res 2007;20:345-59.
3. Whitton ME, Ashcroft DM, Gonz?lez U. Therapeutic interventions for vitiligo. J Am Acad Dermatol 2008;59:713-7.
4. Mahmoud BH, Hexsel CL, Hamzavi IH. An update on new and emerging options for the treatment of vitiligo. Skin Ther Lett 2008;13:1-6.
5. Bahadoran P, Ortonne JP. Classification of surgical therapies for vitiligo. In: GuptaS, OlssonMJ, KanwarAJ, OrtonneJP, editors. Surgical Management of Vitiligo. 1st ed. Massachusettes: Blackwell Publishing Ltd; 2007. p. 69-79.
6. Gupta S, Narang T, Olsson MJ, Ortonne JP. Surgical management of vitiligo and other leukodermas: evidence-based practice guidelines. In: GuptaS, OlssonMJ, KanwarAJ, OrtonneJP, editors. Surgical Management of Vitiligo. 1st ed. Massachusettes: Blackwell Publishing Ltd; 2007. p. 69-79.
7. Mulekar SV. Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol 2004;140:1211-5.
8. Mulekar SV. Melanocyte-keratinocyte cell transplantation for stable vitiligo. Int J Dermatol 2003;42:132-6.
9. Mulekar SV. Long-term follow-up study of 142 patients with vitiligo vulgaris treated by autologous, non-cultured melanocyte-keratinocyte cell transplantation. Int J Dermatol 2005;44:841-5.
10. Mulekar SV, Al Eisa A, Delvi MB, Al Issa A, Al Saeed AH. Childhood vitiligo: A long-term study of localized vitiligo treated by non-cultured cellular grafting. Pediatr Dermatol 2010;27:132-6.
11. Tegta GR, Parsad D, Majumdar S, Kumar B. Efficacy of autologous transplantation of noncultured epidermal suspension in two different dilutions in the treatment of vitiligo. Int J Dermatol 2006;45:106-10.
12. Gauthier Y, Surleve-Bazeille J. Autologous grafting with noncultured melanocytes: a simplified method for treatment of depigmented lesions. J Am Acad Dermatol 1992;26:191-4.
13. van Geel N, Ongenae K, Vander Haeghen Y, et al. Subjective and objective evaluation of noncultured epidermal cellular grafting for repigmenting vitiligo. Dermatology 2006;213:23-9.
14. Van Geel N, Wallaeys E, Goh BK, De Mil M, Lambert J. Long term results of non cultured epidermal cellular grafting in vitiligo, halo nevi, piebaldism and nevus depigmentosus. Br J Dermatol 2010 163(6):1186-93.
15. van Geel NA, Ongenae K, Vander Haeghen YM, Naeyaert JM. Autologous transplantation techniques for vitiligo: how to evaluate treatment outcome. Eur J Dermatol 2004;14:46-51.
16. van Geel N, Ongenae K, De Mil M, et al. Double-blind placebo-controlled study of autologous transplanted epidermal cell suspensions for repigmenting vitiligo. Arch Dermatol. 2004;140:1203-8.
17. van Geel N, Ongenae K, De Mil M, Naeyaert JM. Modified technique of autologous noncultured epidermal cell transplantation for repigmenting vitiligo: a pilot study. Dermatol Surg 2001;27:873-6.
18. Pandya V, Parmar KS, Shah BJ, Bilimoria FE. A study of autologous melanocyte transfer in treatment of stable vitiligo. Indian J Dermatol Venereol Leprol 2005;71:393-7.
19. Szabad G, Kormos B, Pivarcsi A, et al. Human adult epidermal melanocytes cultured without chemical mitogens express the EGF receptor and respond to EGF. Arch Dermatol Res 2007;299:191-200.
20. El-Zawahry BM, Zaki NS, Bassiouny DA, Sobhi RM, Zaghloul A, Khorshied MM, et al. Autologous melanocyte-keratinocyte suspension in the treatment of vitiligo. J Eur A cad Dermatol Venereol 2011;25:215-20
21. Njoo MD, Nieuweboer-Krobotova L, Westerhof W. Repigmentation of leucodermic defects in piebaldism by dermabrasion and thin split-thickness skin grafting in combination with minigrafting. Br J Dermatol. 1998;139:829-833.
22. Olsson M, Juhlin L. Leucoderma treated by transplantation of a basal cell layer enriched suspension. Br J Dermatol 1998;138:644-8.
23. Sahni, K., Parsad, D., Kanwar, A. J. and Mehta, S. D. (2011), Autologous Noncultured Melanocyte Transplantation for Stable Vitiligo: Can Suspending Autologous Melanocytes in the Patients' Own Serum Improve Repigmentation and Patient Satisfaction?. Dermatologic Surgery 2011; 37: 176-182.
24. Khunger N. Thin split-thickness skin grafts for vitiligo. In surgical management of vitiligo. In: Gupta S, et al ., editors. Blackwell Publishing Ltd; 2007. p. 108-14
25. Farah Sameem, Sheikh Javeed Sultan, and Qazi Masood Ahmad. Split Thickness Skin Grafting in Patients with Stable Vitiligo. J Cutan Aesthet Surg. 2011 Jan-Apr; 4(1): 38-40.
26. Khunger N, Kathuria SD, Ramesh V. Tissue grafts in vitiligo surgery - past, present, and future. Indian J Dermatol 2009;54:150-8
27. Srinivas CR, Rai R, Kumar PU. Meshed split skin graft for extensive vitiligo. Indian J Dermatol Venereol Leprol 2004;70:165-7
28. Al-Mutairi N, Manchanda Y, Al-Doukhi A, Al-Haddad A. Long-term results of split-skin grafting in combination with excimer laser for stable vitiligo. Dermatol Surg. 2010;36:499-505.
29. Loentz W , Olsson M & Moellmann G . Pigment cell transplantation for treatment of vitiligo . J Am Acad Dermatol 1994; 30 : 591 - 7 .
30. Austin LM, Boissy RE, Jacobson BS, Smyth JR Jr. The detection of melanocyte autoantibodies in the Smyth chicken model for vitiligo. Clin Immunol Immunopathol 1992;64:112-20.
31. Olsson MJ, Juhlin L. Repigmentation of vitiligo by transplantation of cultured autologous melanocytes. Acta Derm Venereol 1993;73:49-51.
32. Back C, Dearman B, Li A, Neild T, Greenwood JE. Non-cultured keratinocyte/melanocyte cosuspension: Effect on reepithelialization and repigmentation--a randomized, placebo-controlled study. J Burn Care Res 2009;30:408-16.
33. Kachhawa D, Kalla G. Keratinocyte-melanocyte graft technique followed by PUVA therapy for stable vitiligo. Indian J Dermatol Venereol Leprol 2008;74:622-4.
34. Mulekar SV, Ghwish B, Al Issa A, Al Eisa A. Treatment of vitiligo lesions by ReCell vs. conventional melanocyte-keratinocyte transplantation: A pilot study. Br J Dermatol 2008;158:45-9.
35. Cervelli V, De Angelis B, Balzani A, Colicchia G, Spallone D, Grimaldi M. Treatment of stable vitiligo by ReCell system. Acta Dermatovenerol Croat 2009;17:273-8.
36. Xu AE, Wei XD, Cheng DQ, Zhou HF, Qian GP. Transplantation of autologous non-cultured epidermal cell suspension in treatment of patients with stable vitiligo. Chin Med J (Engl) 2005;118:77-9.
37. Goh BK, Chua XM, Chong KL, de Mil M, van Geel NA. Simplified cellular grafting for treatment of vitiligo and piebaldism: The "6-well plate" technique. Dermatol Surg 2010;36:203-7.
38. Lee DY, Choi SC, Lee JH. Comparison of suction blister and carbon dioxide laser for recipient site preparation in epidermal grafting of segmental vitiligo. Clin Exp Dermatol 2010;35:328-9.
39. van Geel, Goh BK, Wallaeys E, De Keyser S, Lambert J. A review of non-cultured epidermal cellular grafting in vitiligo. J Cutan Aesthet Surg. 2011 Jan;4(1):17-22.
40. Mulekar SV, Al Issa A, Al Eisa A. Treatment of vitiligo on difficult-to-treat sites using autologous non-cultured cellular grafting. Dermatol Surg 2009;35(1):66-71.3-12© 2012 Egyptian Dermatology Online Journal |





























