|
|
Introduction
Hidradenitis Suppurativa (HS)
Synonyms: Apocrinitis, Hidradenitis Axillaries, Acne Inversa
It was first described in 1885 by French physician Alfred Verneuil for
its characteristic intertriginous distribution [1].
It is a recurrent and suppurative disease with an insidious onset. It is
characterized by deep furuncles, abscesses, fistulas, sinus tracts and scarring
and has a prevalence rate of 0.3% to 4% of the general population [2].
The average age of onset is 23 years but it may fall anywhere between post-pubertal
age to middle age [3]. HS is more prevalent
in women than men by a ratio of between 2:1 and 5:1, with genito-femoral
lesions found more often in women and anogenital lesions found more often
in men [3,4].
The most commonly affected site, the axilla, is involved in equal proportion
of men and women. Other affected area may include the areola, infra-mammary
folds, periumbilical skin, scalp, zygomatic and malar face, buttocks, thighs,
popliteal fossa, ear canal and eyelids [2,5].
Patients with active disease generally develop 2 furuncles per month with
average disease duration of 19 years [5].
Case report
A 42yr old male presented to dermatology department with complaint of
generalized, bilaterally symmetrical, mildly pruritic, reddish tender lesions
on the face, shoulder and back since last 5 years. The patient also complained
of patchy hair loss following the appearance of painful lesions on the scalp
since last 4 years. There was history of large painful pus filled lesions
with purulent discharge in the axillary and anogenital regions since last
3-4 years for which patient had been consulting a general surgeon. He had
taken multiple courses of several antibiotics for the lesions which improved
partially, only to recur later. He had undergone incision and drainage for
the lesions around the anus following which pus discharge, pain and tenderness
decreased after 2 days, but relapse occurred after 15 days. He gave a history
of a dimple at the lower end of the spine. Patient had, however, never ever
taken any treatment for the lesions on scalp and the back. He was a chronic
smoker since last 20 years. There was no history of joint pain or joint
swelling. He had no history of exposure to chemicals due to occupation or
otherwise. There was no history of diabetes. Family history for similar
disease was negative.
Physical examination:
The patient was overweight but otherwise healthy. Vitals were normal.
Dermatological examination revealed few tender papules with patchy areas
of scarring alopecia (on the vertex) on the scalp. Patient also had male
pattern hair loss. There were generalized bilaterally symmetrical multiple
follicular papules, follicular pustules and 2 to 3 cm fluctuating nodules
on the face, shoulder and back. There were multiple atrophic scars on the
face and more on the back. There were multiple, intermingled, 2 to 6 cm,
at places linear hypertrophic and puckered scars and dermal contractures
in the axillae and the anogenital region.
Scarring, fibrosis and sinus tracts were seen. The lesions were painful
and had a foul odour.
Investigations:
Cultures of the lesions did not grow out organisms. Haemoglobin was 10.5
gm%, Total Leucocyte Count was 10,500/ mm3, Differential Leucocyte Count
was Neutrophil 73%, Lymphocyte 23%, Esnophils 2% and Monocyte 2%. Erythrocyte
Sedimentation Rate was 30 mm at the end of first hour. Total Serum Protein
was 7 gm%, Albumin 4 gm% and Globulin 3 gm%. Repeated cultures of pus, Rheumatoid
Factor, Lupus Erythematosus cell phenomenon, Mantoux test were negative.
Urine and stool tests were normal. Patient was non reactive for Human Immuno
Deficiency Virus and Hepatitis B Surface Antigen (HBsAg).
In the face of typical clinical picture the diagnosis of follicular occlusion
tetrad was made which includes four entities namely Acne Conglobata, Hidradenitis
Suppurativa, Dissecting Cellulitis of scalp and Pilonidal Sinus.
Treatment:
Patient was started on topical clindamycin lotion twice daily and oral
clindamycin and rifampicin 300 mg each twice daily for 12 weeks. Patient
was reviewed every fortnight. At the end 3 months of above therapy patient
responded partially. So keeping in view his clinical presentation of Follicular
Occlusion Tetrad (FOT) along with male pattern baldness he was started on
tab finasteride 5 mg daily while continuing the topical therapy. The patient
started improving on this treatment. At the end of three months the lesions
became quiescent and there was no relapse till 1 year thereafter.
Discussion
The initial symptoms of HS are localized erythema, pruritus and hyperhidrosis
followed by the spontaneous development of painful and tender red nodules
and abscesses with incessant purulent drainage, open comedones, sinus tracts
and scarring [2,4].
This was present in our patient.
Obesity tends to be more prevalent in patients with HS [6],
although it is not been universally demonstrated in all studies [7].
It has been reported that the Body Mass Index (BMI) for men and women with
HS is elevated at 27.8(+3) and 28.5(+5), respectively [8].
Obesity acts as a secondary factor, aggravating disease through mechanical
trauma at skin folds. Our patient was overweight.
Our patient was a chronic smoker of 20 yrs. A high incidence of smoking
(89% are active smokers compared with 46% of controls) occurs amongst HS
patients [9,10].
While HS is not typically thought to be related to medications, it has been
reported to be exacerbated by lithium [11,12]
and sirolimus [13].
Family history for similar disease was negative. In women, HS occurs
almost exclusively after menarche. Premenstrual flares are common in about
50% of all cases. HS tends to improve with pregnancy and rebounds after
parturition. Signs of virilization have been seen in HS but most case series
indicate no significant change in serum hormone levels in HS patients [14].
The free androgen index is increased because of low level of sex-hormone-binding
globulin, which is influenced by body weight. Thus, the role of androgens
in the pathogenesis of HS remains unclear and proved to be secondary rather
than primary.
Familial forms of HS have been described, although no specific gene has
been identified [15]. A recent genome-wide
scan in a Chinese family showed that a disease gene of acne inversa is located
on chromosome 1p21.1-1q25.3 [16].
HS has been associated with various dermatologic syndromes including
Keratitis Ichthyosis Deafness [17] which
was absent in our patient, Synovitis-Acne-Pustulosis-Hyperostosis-Osteitis
syndrome [18], and Crohns disease [19]
in addition to the follicular triad. Arthritis is commonly associated with
HS and can worsen with a flare of HS. Arthropathy typically involves the
large joints in the extremities, particularly the knee joints. There was
no history of joint pain or joint swelling.
Chronic HS lesions have a higher risk of developing malignancy [20].
HS has a significant negative effect on quality of life that correlates
with the disease severity and a more detrimental effect than other dermatologic
conditions [21].
Hurley classification [22] separates
HS into three stages: Stage I is limited to the presence of abscesses without
evidence of sinus tracts or scarring. Stage II demonstrates sinus tracts
and scarring with discrete recurrent abscesses. Stage III represents diffuse
involvement of interconnected sinus tracts, scars and abscesses. Prominent
open comedones often with multiple orifices are seen. The more severe the
disease, the more refractory it is to medical treatment and more likely
it is to recur after surgical intervention. Our patient was in Stage II.
HS lesions are concentrated where apocrine hair follicles are typically
found. The mechanism of disease for HS is not completely understood and
has evolved from being a process centered on apocrine glands [23]
to a process centered on hair follicles. While inflammation of the apocrine
glands can be seen, the patho-physiology of HS is thought to be similar
to acne vulgaris, with hair follicle occlusion being the initiating event.
The disease begins with spongiosis of the infrainfundibular region; follicular
hyperkeratosis and dilatation of follicular infundibula, leading to comedone
formation and follicular rupture [24].
Follicular rupture induces the recruitment first of neutrophils, followed
by a granulomatous infiltrate with foreign body giant cells [24].
The dermal abscesses then extends into the subcutaneous fat to involve the
adenexal structures. Thus, the inflammation of the apocrine glands, or hidradenitis,
is thought to be a secondary event [25].
The subsequent fibrosis and formation of sinus tracts are likely a result
of tissue repair response to chronic inflammation, bacterial superinfection,
and necrotic debris[26]. HS is thus a misnomer,
because the pathogenesis does not involve sweat glands dysfunction as originally
thought.
Whereas the role of infection is not clearly understood, though multiple
species of bacteria are isolated from HS lesions. At times, cultures are
routinely negative. Thus bacterial infection is likely to be secondary to
chronic sinus tracts and moisture rather than of primary etiologic importance.
When present, bacterial cultures are often polymicrobial. Staphylococcus
aureus, S epidermidis, Streptococcus milleri and S hominis have all been
isolated from aspiration of deep lesions about 49% of the time [27].
Peptostreptococcus was the most common anaerobic organism. Enterococcus,
Enterobactericiae, diptheroids, Bacillus cereus, Proprionibacterium acnes,
Lactobacillus and Bacteroides have also been described [28].This
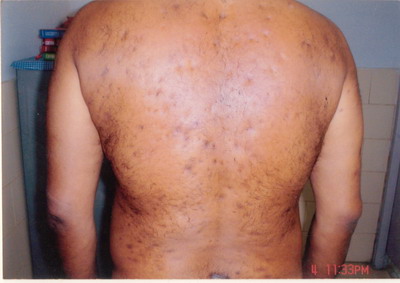
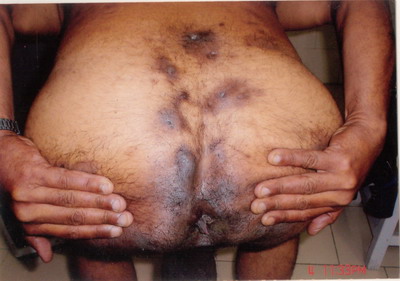
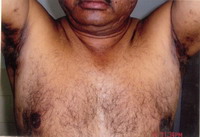
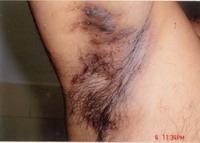
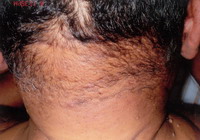
case is being reported for the complete Tetrad of HS (Fig. 1), Dissecting
Cellulitis (Fig. 2), Acne Conglobata (Fig. 3), And Pilonidal
Sinus (Fig. 4), are known as the Follicular Occlusion Tetrad.
|
|
|
| Fig 1: Hidradenitis suppurativa |
|
|

|
|
| Fig 2: Dissecting Cellulitis |
|
Treatment:
Treatment of HS is challenging. Although there are various treatment
options for HS, none of the traditional therapies is wholly satisfactory
or uniformly effective. Therapy varies based on the on the clinical severity
of the disease, although there is considerable overlap between treatment
groups. Mainstays of treatment are non-steroidal anti-inflammatory drugs
for pain and inflammation, antiseptics, antibacterial soaps, warm compresses,
hydrotherapy, and antibiotics.
Medical treatments can reduce inflammation and associated tenderness
and drainage, but usually do not halt disease progression. Medical therapy
can be divided into topical and systemic. Clindamycin 1% solution twice
daily for 12 weeks helps in resolving abscesses and pustules [29].
Systemic medications can be further divided into antibiotics, retinoids,
hormonal modulators, and immunosuppressants. Antibiotics can be helpful,
particularly if specific organisms can be demonstrated. However, antibiotics
require fairly long courses of therapy. Oral tetracyclines, clindamycin,
rifampicin and minocycline have been tried. In a case series of 14 patients
receiving oral clindamycin 300 mg twice daily and rifampicin 300 mg twice
a day for 10 weeks achieved remission in 8 of the 14 patients. Remission
was induced in 2 additional patients when minocycline was substituted for
clindamycin[30]. Results from systemic
retinoids, particularly isotretinoin, have proven to be disappointing. A
prospective trial with long term follow-up showed that only 23% of HS patients
benefit from isotretinoin 0.5 to 1 mg/kg and only 16% of patients showed
durable remission after treatment[31].
Antiandrogen therapy has been studied in HS. Cyproterone acetate and
spironolactone need further study to define their role in treating HS. Finasteride,
a 5-alpha reductase inhibitor, 5 mg once a day showed improvement by 8 weeks
and remissions from 8 to 18 months [32].
Immunosupressive therapy has been used in HS particularly in acute inflammatory
stage with varying degrees of success. Dapsone, cyclosporine, azathioprine,
mycophenolate mofetil, tacrolimus have been tried.
Biologicals like TNF-alpha inhibitors (infliximab, etarnecept, adalimumab)
and T-cell specific agents (alefacept and efalizumab) for the treatment
of HS have thus far proved to have variable long-term results and are laden
with significant adverse effects. However, the use of three anti-TNF biologic
agents might be considered a potentially life-altering maneuver [33,34,and35].
Non-surgical modalities like cryotherapy, carbon-dioxide laser and photodynamic
therapies have been tried but none of them have proved to be satisfactory.
Radiotherapy with x-rays has been reported to be useful in control of the
disease [36].
Surgical excision is the treatment of choice for early disease. Surgical
techniques for excision and repair vary, depending on extent of disease,
location of the lesions, existing comorbidities, and chronicity of disease
[37].
Differential Diagnosis:
Possible misdiagnoses are numerous, and vary with the stage and site.
Axillary and inguinal lesions that have ulcerated may be confused with scrofuloderma.
Inguinal lesions may simulate actinomycosis, granuloma inguinale or lymphogranuloma
venereum. When only one or a few nodules and sinuses are present in the
anogenital region, pilonidal sinus, sigmoidal diverticulitis and Crohn's
disease must be excluded.
In atypical cases, the diagnosis may be difficult. The characteristic
comedones should be sought and the non-specific histological changes may
be negatively useful.
References
1. Verneuil A. NonEnl'Hidrosadenite Phlegmoneuse Et Des
Abces Sudoripares. Arch Gen Med 1864;24:537-57.
2. Brown TJ, Rosen T, Orengo IF. Hiradenitis suppurativa.
South Med J 1998;91:107-14 .
3. Wiseman MC. Hidradenitis suppurativa: a review. Dermatol
Ther 2004;17;50-4 .
4. Krebc AC. Current understanding and management of hiradenitis
suppurativa. J Am Acad Nurse Pract 2007;19:228-34 .
5. Ather S, Chan DS, Leaper DJ et al. Surgical treatment
of hiradenitis suppurativa: case series and review of literature. Int Wound
J 26;3:159-169 .
6. Edlich RF, Silloway KA, Rodeheaver GT, et al. Epidemiology,
pathology and treatment of axillary hidradenitis suppurativa. J Emerg Med
1986;4:369-78 .
7. Jemec GB, Heidenheim M, Nielsen NH. Hidradenitis suppurativa-characteristics
and consequences. Clin Exp Dermatol 1996;21:419-23 .
8. Harrison BJ, Read GF, Hughes LE. Endocrine basis for
the clinical presentation of hidradenitis suppurativa. Br J Surg 1988;75:972-5
.
9. Konig A, Lehmann C, Rompel R, et al. Cigarette smoking
as a triggering factor of hidradenitis suppurativa. Dermatology 1999;198:261-4
.
10. Freiman A, Bird G, Metelista AI, et al. Cutaneous effects
of smoking. J Cutan Med Surg 2004;8:415-23 .
11. Marinella MA. Lithium therapy associated with hidradenitis
suppurativa. Acta Derm Venereol 1997;77:483 .
12. Gupta AK, Knowles SR, Gupta MA, et al. Lithium therapy
associated with hidradenitis suupurativa: case report and a review of the
dermatologic side effects of lithium. J Am Acad Dermatol 1995;32:382-6 .
13. 13. Mahe E, Morelson E, Lechaton S, et al. Cutaneous
adverse events in renal transplant recipients receiving sirolimus-based
therapy. Transplantation 2005;79:476-82 .
14. Harrison BJ, Kumar S, Read GF, et al. Hidradenitis
suppurativa : evidence for an endocrine abnormality. Br J Surg 1985;72:1002-4
.
15. Von Der Werth JM, Williams HC, Reburn JA. The clinical
genetics of hidradenitis suppurativa revisited. Br J Dermatol 2000;142:947-53
.
16. Gao M, Wang PG, Cui Y, et al. Inversa acne (hidradenitis
suppurativa) : a case report and identification of the locus at chromosome
1 p21. 1-1q25.3. J Invest Dermatol 2006;126:1301-6 .
17. Manitz L, Betz RC, Allam JP, et al. Keratitis-ichthyosis-deafness
syndrome in association with follicular occlusion triad. Eur J Dermatol
2005;15:347-52 .
18. Ozyemisci-Taskiran O, Bolukbasi N, Gogus F. A hidradenitis
suppurativa related SAPHO case associated with features resembling spondylartropathy
and proteinuria. Clin Rheumatol 2006;5(5):789-91 .
19. Attanoos RL, Appleton MA, Hughes Le, et al. Granulomatous
hidradenitis suppurativa and cutaneous Crohn's disease. Histopathology 1993;23:111-5
.
20. Lapins J, Ye W, Nyren O, et al. Incidence of cancer
among patients with hidradenitis suppurativa. Arch Dermatol 2001;137:703-4
.
21. Von der Werth JM, Jemec GB. Morbidity in patients with
hidradenitis suppurativa. Br J Dermatol. 2001;144:809-13 .
22. Hurley H. Axillary hyperhidrosis, apocrine hyperhidrosis,
hidradenitis suppurativa, and familial benign pemphigus. In : Roegnick RH,
Roegnick HH, editors. Dermatologic surgery. New York : Marcel Dekker; 1989.
P. 717 -43 .
23. Shelley WB, Cahn MM. The pathogenesis of hidradenitis
suppurativa in man; experimental and histologic observations. AMA Arch Derm
1955;72:562-5 .
24. Boer J, Weltevreden EF. Hidradenitis suppurativa or
acne inversa. A clinicopathological study of early lesions. Br J Dermatol
1996;135:721-5 .
25. Jemec GB, Thomsen BM, Hansen U. The homogeneity of
hidradenitis suppurativa lesions. A histological study of intra-individual
variation. Apmis 1997;105:378-83 .
26. Attanoos RL, Appleton MA, Douglas-Jones AG. The pathogenesis
of hidradenitis suppurativa: a closer look at apocrine and apoeccrine glands.
Br J Dermatol 1995;133:254-8 .
27. Jemec GB, Faber M, Gutschik E, et al. The bacteriology
of hidradenitis suppurativa. Dermatology 1996;193:203-6 .
28. Lapins J, Jarstrand C, Emtestam L. Coagulase-negative
staphylococci are the most common bacteria found in cultures from the deep
portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide
laser surgery. Br J Dermatol 1999;140:90-5
29. Clemmensen OJ. Topical treatment of hidradenitis suppurativa
with clindamycin. Int J Dermatol 1983; 22: 325-8.
30. Mendonca CO, Griffiths CE. Clindamycin and rifampicin
combination therapy for hidradenitis suppurativa. Br J Dermatol 2006;154
: 977-8.
31. Boer J, van Gemert MJ. Long -term results of isotretinoin
in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad
Dermatol 1999;40:73-6.
32. Joseph MA, Jayaseelen E, Ganapath B, et al. Hidradenitis
suppurativa treated with finasteride. J Dermatolog Treat 2005;16:75-8.
33. Lebwohl B, Sapadin AN. Infliximab for the treatment
of hidradenitis suppurativa. J Am Acad Dermatol 2003;49:S275-6.
34. Cusack C, Buckley C. Etanercept: effective in the management
of hidradenitis suppurativa. Br J Dermatol 2006;154:726-9.
35. Moul DK, Karman NJ. The cutting edge. Severe hidradenitis
suppurativa treated with adalimumab. Arch Dermatol. 2006;142:1110-2.
36. Zeligman I. Temporary x-ray epilation therapy of chronic
axillary hidradenitis suppurativa. Arch Dermatol 1965;92:690-4.
37. Kagan RJ, Yakuboff KP, Warner P, et al. Surgical treatment
of hidradenitis suppurativa: a 10-year experience. Surgery 2005;138:734-40.
© 2012 Egyptian Dermatology Online Journal
|