|
|
Abstract
Background: Psoriasis is a chronic inflammatory hyper-proliferative disease
of the skin, scalp, nails, and joints. It has been hypothesized that prolactin
(PRL) may modulate the skin immune system and may be involved in the pathogenesis
of psoriasis. Psoriasis exerts significant, negative impact on patients'
quality of life. Relatively high rates of depression are reported in patients
with psoriasis.
Objectives: The aim of this work was to study the possible role of PRL
in the pathogenesis of psoriasis and its correlation with the disease activity
and emotional status of the patients.
Subjects and methods: This study included 30 patients with chronic generalized
psoriasis and 10 healthy subjects served as controls, attending the Outpatient
Clinic of Dermatology and Venereology Department, Tanta University Hospital.
Serum samples were taken from all patients before and after 4 weeks of treatment
with 25 mg / week of methotrexate (MTX) (I.M injection) for detection of
PRL serum levels by ELISA, while serum samples were taken from control subjects
once for comparison. Psychological instruments; [The Hamilton Depression Scale(HAMD) and The Hamilton Anxiety Scale(HAMA)]
were used to screen the broad range of psychological factors that have been
implicated in psoriasis.
Results: Serum PRL levels were statistically highly significant among
patients compared to controls, with significant decrease among patients
after treatment. A significant positive correlation was found between PRL
serum levels and PASI score before and after treatment. Correlations between
HAMA and HAMD with PASI were statistically significant before and after
therapy, and so the correlations between HAMA and HAMD with PRL serum levels
were also statistically significant before and after therapy.
Conclusion: Prolactin seems to have a role in the pathogenesis of psoriasis.
This role may represent a cause and/or a consequence of psoriasis pathology.
The most likely scenario is that PRL enhances interferon-induced chemokine
production in keratinocytes, thereby facilitating cutaneous T-cell infiltration.
This raises the intriguing prospect that PRL may offer a novel future therapeutic
target in psoriasis and other skin diseases that worsen in response to psychological
distress.
Introduction
Psoriasis is an inflammatory disease characterized by hyper-proliferation
of keratinocytes and accumulation of T cells in the epidermis and dermis
of psoriatic lesions. [1] Evidence for the
central role of T helper (Th1) lymphocytes comes from both animal models
of psoriasis and from trials of treatment with T-cell inhibitors. [2]
There is some evidence that psoriasis worsens at ages when hormonal changes
such as puberty and menopause are taking place, and may also worsen or improve
during pregnancy. [3]
Prolactin (PRL), a neuropeptide secreted by the anterior pituitary gland,
possesses a variety of physiological actions. It has been implicated as
an important immuno-modulator and exerts a proliferative effect in cultured
human keratinocytes via specific receptors. Some studies have indicated
an increase in serum PRL levels in psoriasis and exacerbation of psoriasis
when a prolactinoma is present. [4,5]
Emotional disturbances are one of the most important causes of hyper-prolactinaemia,
[6] and stress is significantly associated
with exacerbations of psoriasis, thus secondary hyper-prolactinaemia due
to stress cannot be excluded, nevertheless some authors believe that PRL
may play a role in the pathogenesis of psoriasis. [7,8]
Aim of the work
The aim of this work was to study the possible role of PRL in the pathogenesis
of psoriasis and its correlation with the disease activity and emotional
status of the patients.
Patients and Methods
This study included 30 patients with chronic generalized psoriasis; 24
males & 6 females. Their age ranged from 21-70 years with a mean of 47.833
±12.123 and 10 healthy subjects served as controls, they are age and sex
matched with the studied patients. Their age ranged from 20-66 years with
a mean of 42.100±14.685. They included 7 males and 3 females attending the
Outpatient Clinic of Dermatology and Venereology Department of Tanta University
Hospital during the period from October 2010 to May 2011. The patients were
subjected to:
1- Full history taking:
Including personal history, history of the disease (age of onset, duration
and extension of the disease), past history (history of drug intake, lactation,
menstrual irregularities in females and andrological complaints in males)
and family history.
2- Full clinical examination including:
A complete dermatological examination was done for each patient so as
to determine the extent and distribution of the disease .Clinical severity
of psoriasis was assessed by using the Psoriasis Area Severity Index (PASI)
score. Points for erythema, infiltration and desquamation of the skin ranged
from 1 to 4, and the involved area from 1 to 6, thus theoretically the PASI
ranges from 0 to 72, with higher scores indicating more severe condition.
In our study mild psoriasis was classified as a PASI less than 7, moderate
psoriasis as a PASI between 7 and15, and severe psoriasis as a PASI of >15.
[9]
The following patients were excluded from the study:-
-Hepatic and renal patients, pregnant or lactating females, patients
who are receiving any medications affecting PRL such as phenothiazines (chrompromazine),
antidopaminergic agents (metaclopromide), antihypertensive agents (calcium
channel blockers, methyldopa) and H2 blockers (cimetidine) and endocrinopathies
3- Therapeutic management:
Each patient received 1cm / 25 mg / week of methotrexate (MTX) for 4
weeks (I.M injection).
4- Clinical assessment:
Clinical assessment consisted of clinical response determination after
4 weeks. Photographs of the body sites affected by psoriasis were taken
for each patient before and after the end of treatment. The clinical response
to therapy was scored by using PASI score.
5- Laboratory investigation:
All studied patients were subjected to the following laboratory investigations:-Complete
blood picture, liver and renal function tests, chest and brain x ray and
abdominal Ultrasonography.
Hormonal assay that included serum PRL measured by ELISA technique before
and after the duration of treatment with MTX (4 weeks). The PRL was measured
by using DRG kit supplied by Clinilab ( Louhi-Finland). The specimen was
taken between 8-10AM, blood was collected by veinpuncture, allowed to clot,
the serum separated by centrifugation at room temperature and stored at
20ºC until used. 25μl of each standard, control and samples were dispensed
into appropriate wells,100 μl Enzyme Conjugate was dispensed into each well,
and incubated for 30 minutes at room temperature, then the wells was rinsed
5 times with distilled water,100 μl of Substrate Solution was added to each
well and was incubated for 10 minutes at room temperature, the enzymatic
reaction was stopped by adding 50 μl of Stop Solution to each well.[10]
6- Specific and generic measures of psychological distress:
The psychological instruments were as follows:
-The Hamilton Depression Scale (HDS, HAMD or HAD) is a depression test
measuring the severity of clinical depression symptoms. It consists of 21
items, each defined by series of symptoms. Some items were rated on a 5-points
scale, ranging from 0 (not present) to 4 (severe), another items were rated
on a 3-points scale, ranging from 0 (not present) to 2 (severe). The scoring
is based on the first 17(0-7 = Normal, 8-13 = Mild Depression, 14-18 = Moderate
Depression, 19-22 = Severe Depression,≥23 = Very Severe Depression). [11]
- The Hamilton Anxiety Scale (HAMA) is a rating scale developed to quantify
the severity of anxiety symptomatology. It consists of 14 items, each defined
by a series of symptoms. Each item is rated on a 5-point scale, ranging
from 0 (not present) to 4 (severe), with a total score range of 0-56, where
<17 indicates mild severity, 18-24 mild to moderate severity and 25-30 moderate
to severe. [12]
The questions of the 2 questionnaire were asked to the patients before
and after the duration of the treatment with MTX.
Statistics
Statistical presentation and analysis of the present study was conducted,
using the mean, standard error, student t- test and Chi-square, by SPSS
V17. Statistical significance was determined at a level of P < 0.05.
Results
Clinical results:
In this study the duration of the psoriasis ranged from 2 years to 43
years, with a mean of 14.0 ±12.382 years. As regards family history, 2 out
of the 30 patients had positive family history (6.67%). According to PASI
score, 7 patients (23.33%) were classified as moderate psoriasis, 6 of them
became mild (Fig 1) and 1 remained moderate after therapy, 23 patients
(76.67%) were classified as severe psoriasis, 8 of them became mild, 9 became
moderate (Fig 2) and 6 remained severe after therapy. There is a significant
decrease in the value of PASI before and after therapy (Table 1).
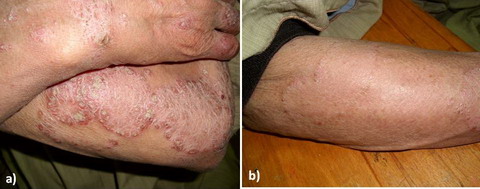
Fig 1: a) Moderate psoriasis before treatment
[PASI=12.4]
b) Mild
psoriasis after treatment
[PASI=2] |
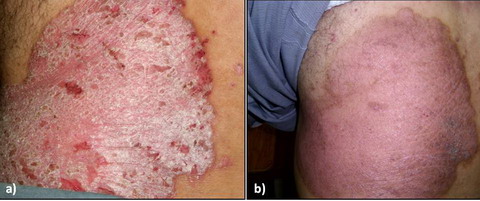
Fig
2: a) Severe psoriasis before treatment
[PASI=39.8]
b) Moderate
psoriasis after treatment
[PASI=15] |
| PASI score before ttt |
PASI score after ttt |
| Mild |
Moderate |
Severe |
Total |
| Moderate |
N |
6 |
1 |
0 |
7 |
| % |
20 |
3.33 |
0 |
23.33 |
| Severe |
N |
8 |
9 |
6 |
23 |
| % |
26.67 |
30 |
20 |
76.67 |
| Total |
N |
14 |
10 |
6 |
30 |
| % |
46.67 |
33.33 |
20 |
100 |
| Chi-square |
X2 |
6.973 |
| P-value |
0.031* |
ttt: treatment *: significant
Table
Table (1): The values of PASI score before and after therapy.
Laboratory results:
Serum levels of PRL in the group of patients ranged from 31.5 ng/ml to
80.6 ng/ml with a mean and SD of 49.590 ng/ml ± 15.473 in comparison with
controls that ranged from 7-20 ng/ml with a mean and SD of 14.00 ± 5.228,
so the cutoff point between control group and patients group is 20 ng/ml (Fig 3). There is statistically highly significant increase among patients
(P=0.000). As regards the serum levels of PRL after therapy, they ranged
from 9.0 to 40.0 ng/ ml with a mean and SD of 20.553 ± 8.276 with significant
decrease in comparison with values before treatment (Table 2).
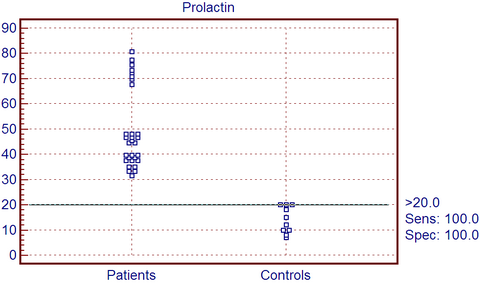
| Fig
3: Cut off point between patients and control as regard PRL. |
| |
Prolactin |
Paired T-test |
| Range |
Mean |
± |
SD |
T |
P-value |
| Before ttt |
31.5 |
- |
80.6 |
49.59 |
± |
15.473 |
|
|
| After ttt |
9 |
- |
40 |
20.553 |
± |
8.276 |
15.823 |
0.000* |
Table (2): Serum PRL levels in psoriasis patients before and
after therapy.
Psychological results:
The values of HAMA score before therapy ranged from 3.0 to 29.0 with
a mean and SD of 13.733 ± 6.938, while after therapy the values significantly
decreased, they ranged from 1.0 to 18.0 with a mean and SD of 7.600 ± 5.076
(p=0.000) (Table 3). The values of HAMD score before therapy ranged from
2.0 to 23.0 with a mean and SD of 9.700 ± 6.353, while after therapy the
values significantly decreased, they ranged from 1.0 to 18.0 with a mean
and SD of 5.100 ± 3.772 (p=0.000) as shown in (Table 3).
| |
HAMA |
Paired T-test |
| Range |
Mean |
± |
SD |
t |
P-value |
| Before ttt |
3 |
- |
29 |
13.733 |
± |
6.938 |
|
|
| After ttt |
1 |
- |
18 |
7.6 |
± |
5.076 |
8.279 |
0.000* |
| |
HAMD |
Paired T-test |
| Range |
Mean |
± |
SD |
t |
P-value |
| Before ttt |
2 |
- |
23 |
9.7 |
± |
6.353 |
|
|
| After ttt |
1 |
- |
18 |
5.1 |
± |
3.772 |
7.367 |
0.000* |
Table (3): Comparison between the values of HAMA and HAMD scores
before and after therapy. Correlations between HAMA and HAMD with PASI and PRL before therapy were
statistically significant (Table 4).Correlations between PRL with HAMA,
HAMAD and PASI were statistically significant after therapy (Table 5)..
| Correlations |
PASI before ttt |
PRL |
HAMA before ttt |
| Before ttt |
| HAMA before ttt |
r |
0.848 |
0.822 |
|
| P-value |
0.000* |
0.000* |
|
| HAMD before ttt |
r |
0.918 |
0.9 |
0.794 |
| P-value |
0.000* |
0.000* |
0.000* |
Table (4): The correlation between HAMA and HAMD with PASI and
PRL before therapy.
| Correlations |
PASI after ttt |
Prolactin after ttt |
HAMA after ttt |
| Prolactin after ttt |
r |
0.923 |
|
|
| P-value |
0.000* |
|
|
| HAMA after ttt |
r |
0.839 |
0.835 |
|
| P-value |
0.000* |
0.000* |
|
| HAMD after ttt |
r |
0.918 |
0.808 |
0.816 |
| P-value |
0.000* |
0.000* |
0.000* |
Table (5): The correlation between PRL with PASI, HAMA and
HAMD, and the correlation between HAMA and HAMD with PASI after therapy
Discussion
Stress and hormonal factors have been reported to be implicated in the
pathogenesis of psoriasis. [13] It has
been hypothesized that PRL may modulate the skin immune system and may be
involved in the pathogenesis of psoriasis. Functional PRL receptors are
detected on keratinosis and PRL effectively increased the in vitro growth
of keratinocytes in psoriasis. [14] PRL
acts as a neuroendocrine modulator of both skin epithelial growth and the
skin immune system. It is hypothesized to be integrated in a multilevel
neuroendocrine-immune communication along the ''brain-skin axis''. Stress
has been reported to trigger and exacerbate psoriasis; therefore, it may
represent a link between prolactin and the disease pathogenesis. [15]
So, the aim of this study was to study the possible role of PRL in the pathogenesis
of psoriasis and its correlation with the disease activity and emotional
status of the patients.
Our results revealed that serum level of PRL was significantly higher
in psoriatic patients than controls and were significantly reduced after
treatment and there was a correlation between serum PRL levels and PASI.
These results are consistent with those of Dilme-carreras et al. [8],
their study included 20 patients of psoriasis, and 20 healthy controls.
PRL levels were measured by Enzyme- linked Immuno Assay (EIA) in serum of
patients of psoriasis before and after treatment with tacalcitol ointment
4μ / g once a day for 6 weeks and disease severity was assessed by PASI
in all patients. Serum levels of PRL were significantly higher in patients
than controls before treatment. They also found that PRL levels were significantly
reduced after treatment with tacalcitol and there was a correlation between
pretreatment serum PRL levels and PASI. Also in a study done by El-Khateeb
et al. [16], who initiated a study including
15 psoriasis patients and 15 healthy volunteers as controls, PASI score
was evaluated, and PRL levels in serum and blister fluid were assessed by
(ELISA). PRL levels were significantly elevated in blister fluid of psoriatic
lesional skin. Correlations between PASI score and different serum PRL levels
in lesional and non-lesional skin were insignificant. Significant positive
correlations of prolactin level were observed between lesional and non-lesional
skin in psoriasis and between serum and clinically normal skin in both psoriasis
and control subjects.
Regana and Millet, 2000 reported three cases of women with plaque-type
psoriasis that severity and extent of the skin lesions correlated with development
of a prolactinoma [17]. All three patients
were treated with bromocriptin. They had normalization of PRL level and
also improvement of psoriatic lesions. Then, they discontinued bromocriptin
and all cases had relapsed in psoriasis. On the other hand Azizzadeh et
al. [14] found that although levels of
serum PRL, which were determined by ELISA in 30 psoriatic patients compared
with 30 controls using Pishtaz Teb kit, has a mean level in psoriatic patients
was not significantly higher than the control group. However, there was
statistically significant relation between severity of disease and serum
prolactin.
Our results revealed also that, values of HAMA and HAMD score were significantly
high in all psoriatic patients, they were significantly decreased after
treatment, and there was a correlation between these values and PASI. These
results are in accordance with Verhoeven et al. [18]
who found a significant association between stress and disease severity.
This prospective study of 62 psoriasis patients determined high levels of
daily stressors to be related to an increase in disease severity 4 weeks
later. In another study carried out by Erick Chern et al. [19],
thirty-six patients with psoriasis receiving conventional treatment and
48 patients receiving the Goeckerman regimen (UV irradiation and coal tar)
were recruited to the study. Clinical severity was evaluated weekly using
PASI. Psoriasis Disability Index (PDI) and Hospital Anxiety and Depression
Scale (HADS) questionnaires were applied at admission and one month after
discharge. The mean PASI score in the Goeckerman group decreased and PDI
scores decreased. HADS scores for anxiety and depression decreased significantly.
In comparison with conventional therapy, the modified Goeckerman regimen
showed similar clinical efficacy, with additional benefits in improving
overall quality of life and psychosocial distress in patients with moderate/severe
psoriasis, and more cost-effectiveness. Unlike the above studies Zachariae
et al. [20] showed that stress responders
differed significantly from non-stress responders. Stress responders tended
to self-report greater disease severity than non-stress responders, even
though clinical measures of disease severity (e.g. PASI) did not vary between
groups. Stress responders were, however, found to have more plaques of psoriasis
on visible areas than non-visible regions.
The PRL may be locally produced in the skin, and such production revealed
a relatively marked increase in psoriatic lesions. The cutaneous contribution
of PRL may be diluted in the circulation before it reaches a threshold from
which it can begin to influence the serum level of PRL, accordingly, it
seems to have a role in the pathogenesis of psoriasis. This role may represent
a cause and/or a consequence of psoriasis pathology. [16]
There was a study involved 10 patients with early-onset chronic plaque psoriasis
and 10 controls, skin biopsies were taken from all of them, that were subsequently
analyzed immuno-histochemically. Both PRL and prolactin receptors were detected
in epidermal keratinocytes, dermal fibroblasts, and sweat glands of healthy
controls. By comparison, however, expression of PRL and PRL receptors was
markedly up-regulated throughout the epidermis in psoriasis plaques, especially
in the basal layer, probably because of local cutaneous production. No significant
difference was noted between expression in the uninvolved skin of psoriasis
patients and samples from normal controls. [21]
Prolactin enhances proliferation of IFN-g production in T cells or natural
killer cells and potentiates IFN-g -induced chemokine production in keratinocytes
which, in turn, generates abundant infiltrates of type I T cells. It stimulates
the hyper-proliferation of keratinocytes and induces angiogenesis through
the production of vascular endothelial growth factor. PRL induces CCL20
secretion from epidermal keratinocytes in psoriatic lesions, which recruit
Th17 cells that release IL-17. PRL and IL-17 cooperate in a positive feedback
loop to increase CCL20 secretion. This process may aggravate the Th17-mediated
inflammation in psoriatic lesions. It stimulates antigen-presenting cells
by increasing the expression of MHC class II or co-stimulatory molecules,
CD40, B7-1, or B7-2. [16] Given that cyclosporine
A (CsA) is an effective treatment for psoriasis, [22]
it deserves mentioning that PRL reportedly competes with CsA for a common
binding site on T lymphocytes and that stimulation of PRL secretion reverses
the immuno-suppression induced by CsA. CsA also inhibits PRL-mediated induction
of ornithine decarboxylase, and bromocriptine and CsA have synergistic effects
in the treatment of autoimmune diseases. If PRL can indeed blunt the efficacy
of CsA therapy, this might be countered by co-administration of bromocriptine
or related dopaminergic agents, or by previously unreported PRLR antagonists.
This might allow more effective, but less toxic, lower-dose CsA therapy
in psoriasis. [23] It is possible that
differences in peripheral or lymphocyte, rather than pituitary production
of PRL may worsen disease activity in certain patients. Of the currently
available prolactin-lowering drugs, bromocriptine at least has been shown
to decrease both peripheral and pituitary PRL production and may represent
a useful adjunctive therapy in certain patients, particularly those with
refractory disease. [24]
In conclusion
Prolactin seems to have a role in the pathogenesis of psoriasis. This
role may represent a cause and/or a consequence of psoriasis pathology.
This raises the intriguing prospect that PRL may offer a novel future therapeutic
target in psoriasis and other skin diseases that worsen in response to psychological
distress.
References
1. Griffiths TW, Griffiths CE, Voorhees JJ. Immunopathogenesis
and immunotherapy of psoriasis. Dermatol Clin. 1995; 13: 739- 749.
2. Gottlieb AB, Kreuger JC, Wittkowski K et al.Psoriasis
as a model T cell- mediated disease: immunobiologic and clinical effects
of treatment with multiple doses of efalizumab, an anti-CD11a antibody.
Arch Dermatol. 2002; 138: 591- 600.
3. Mowad CM, Margolis DJ, Halperin A et al. Hormonal influences
on woman with psoriasis .Cutis 1998; 61: 257- 260.
4. Buskila D, Sukenik S ,Shoenfeld Y. The possible role
of prolactin in autoimmunity. Am J Repord Immunol. 1991; 26: 118- 123.
5. Girolomoni G, Philips JT, Bergstresser PR. Prolactin
stimulates proliferation of cultured human keratinocytes. J Invest Dermatol.
1993; 101: 275- 279.
6. Cooke NE. Prolactin: normal synthesis, regulation, and
actions. In: Endocrinology. De Groot LJ, (ed). Philadelphia: W.B. Saunders,
1989; pp 384-407.
7. Sánchez Regaña M, Ojeda R , Umbert P.[El impacto psicosocial
de la psoriasis].Actas Dermosifiliogr. 2003; 94: 11- 16.
8. Dilmé-Carreras E, Martin-Ezquerra G, Sánchez-Regaňa
M et al. Serum prolactin levels in psoriasis and correlation with cutaneous
disease activity. Clin Exp Dermatol. 2010; 2: 1- 4.
9. Feldman SR, Krueger GG. Psoriasis assessment tools in
clinical trials. Ann Rheum Dis. 2005; 64: 65- 68.
10. Utolia M, Ruouslahti E, engvall E. Methods. J Immunol.
1981; 42: 11- 15.
11. Williams J. A structured interview guide for the Hamilton
Depression Rating Scale. Arch Gen Psychiatry 1988; 45: 742- 747.
12. Borkovec T, Costello E. Efficacy of applied relaxation
and cognitive behavioral therapy in the treatment of generalized anxiety
disorder. J Clin Consult Psychol. 1993; 61: 611.
13. Damasiewicz-Bodzek A, Kos-Kudła B. [Hormonal factors
in etio-pathogenesis of psoriasis]. Pol Merkur Lekarski 2007; 22: 75- 78.
14. Azizzadeh M, Malek M, Amiri M et al. Does prolactin
indicate severity of psoriasis? Iran J Dermatol. 2009; 12: 79- 81.
15. Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine
circuitry of the ''brain-skin connection''. Trends Immunol. 2006; 27: 32-
39.
16. El-Khateeb EA, Zuel-Fakkar NM, Eid SM et al. Prolactin
level is significantly elevated in lesional skin of patients with psoriasis.
Int J Dermatol. 2011; 50: 693- 696.
17. Sanchez Regana M, Umbert Millet P. Psoriasis in association
with prolactinoma: three cases. Br J Dermatol. 2000; 143: 864- 867.
18. Verhoeven EMW, Kraaimaat FW, de Jong EMGJ et al. Individual
differences in the effect of daily stressors on psoriasis: A prospective
study. Br J Dermatol. 2009; 161: 295- 299.
19. Erick Chern, Diana Yau, Ji-Chen Ho et al. Positive
effect of modified Goeckerman regimen on quality of life and psychosocial
distress in moderate and severe psoriasis. Acta Derm Venereol. 2011; 91:
447- 451.
20. Zachariae R, Zachariae H, Blomqvist K et al. Self-reported
stress reactivity and psoriasis-related stress of Nordic psoriasis sufferers.
J Eur Acad Dermatol Venereol 2004; 18: 27- 36.
21. Jancin B. Prolactin may explain the stress trigger
of psoriasis. Skin & Allergy News.2008; 30.
22. Hijenen DJ, Ten Berge O, Timmer-de Mik L et al. Efficacy
and safety of long-term treatment with cyclosporin A for atopic dermatitis.
J Eur Acad Dermatol Venereol. 2007; 21: 85- 89.
23. Foitzik K, Langan EA, Paus R. Prolactin and the skin:
A dermatological perspective on an ancient pleiotropic peptide hormone.
J Invest Dermatol. 2009; 129: 1071- 1087.
24. Chuang E, Molitch ME. Prolactin and autoimmune diseases
in humans. Acta Biomed 2007; 1: 255- 261.
© 2012 Egyptian Dermatology Online Journal
|



