|
|
Abstract
Background: Parthenium dermatitis is one of the most
common, chronic, relapsing and distressing allergic contact dermatitis
from plants, particularly in India. Steroids have been the mainstay of
treatment. For many years now, various immunosuppressive drugs
particularly Azathioprine, with different dosage schedules are in use.
Aims and objectives: We evaluated the efficacy of
weekly pulse doses of Azathioprine and weekly pulse doses of
Prednisolone in the treatment of Parthenium dermatitis and compared the
two treatment regimens.
Methods: Sixty Parthenium dermatitis patients were
randomly divided into 2 equal groups. Patients of first group were given
oral Azathioprine 300 mg weekly and those of second group oral
Prednisolone 100 mg weekly. Therapy in both groups was given for a total
of 12 weeks with follow up at 4, 8 and 12 weeks.
Results: In the Azathioprine group of patients, the
mean Clinical Severity Score (CSS) at 4, 8 and 12 weeks decreased
respectively to values of 51.7, 39 and 22.3, from the mean value of 66
at 0 week, with statistically significant percentage decline in the mean
of 47.78, 90 and 145.55 respectively. In the Prednisolone group of
patients, the mean CSS at 4, 8 and 12 weeks decreased from 68.3 at 0
week to mean CSS values of 63, 52.3 and 43.3 respectively, with
percentage decline of 17.78, 53.3 and 83.33 respectively. No significant
side effects of the two regimens were detected.
Conclusions: In this preliminary open study, both
Azathioprine in weekly pulse doses and Prednisolone in weekly oral
mini-pulse were found to be effective without any serious adverse
effects in the treatment of Parthenium dermatitis.
Key message: Weekly pulse doses of Azathioprine and
Oral Mini Pulse therapy of steroids can be a safe and effective
alternative to daily dosage regimens of Azathioprine and systemic
steroids.
Limitations: The study group was small and the therapy
was given for 12 weeks only, although therapy with both Azathioprine and
oral mini pulse therapy with steroids is more effective if given for a
prolonged period of time. Follow up was short and Thio Purine Methyl
Transferase (TPMT) level was not done as the facility does not exist.
Introduction
Parthenium hysterophorus [1,2,3,4],
which belongs to family Compositae, is the most common cause of contact
dermatitis from plants in India [5,6,7],
though there is cross sensitivity with other Compositae plants [8,9]
(fig 1).

| Fig 1: Parthenium Hysterophorus plant. |
Various preventive and control measures, eradication of weed,
desensitization of patients with plant material and other treatment
modalities have been tried [10,11,12],
with ineffective, inconsistent and unsuccessful results. The mainstay of
treatment has been the use of local and systemic steroids, which is
associated with several serious and often irreversible side effects [13,14,15,16],
forcing researchers to find modifications in dosage schedules and
alternative therapeutic regimens. Pulse therapy of systemic steroids has
been found to be equally effective to daily regimens, with lesser side
effect profile [17]. Various
immunosuppressive agents like Methotrexate [18],
Cyclosporine [19], Azathioprine have
recently emerged as good therapeutic alternatives to steroids for use in
Parthenium dermatitis. Preliminary studies on the use of Azathioprine, a
potent immunosuppressant in air borne contact dermatitis [20],
actinic reticuloid [21], chronic
actinic dermatitis [22], atopic
dermatitis [23,24]
as well as Parthenium Dermatitis [25,26,27,28,29,30],
have given encouraging and consistently good results, having been used
both as daily [27,3031]
and also weekly pulse dosage schedules [29].
Only few studies have been conducted in India or abroad to prove the
efficacy of weekly pulse of Azathioprine in Parthenium dermatitis. In
the present study, the efficacy of weekly pulse doses of Azathioprine
was evaluated and compared with weekly oral mini pulse therapy of
Prednisolone for the treatment of Parthenium dermatitis, which is quite
distressing ailment for patients and equally disappointing for
dermatologists to manage because of remissions and recurrences
associated.
Materials and Methods
The study was conducted in the Department of Dermatology, Venereology
and Leprosy in a Government Medical College hospital over a period of
one year from November 2007 to October 2008.
Sixty cases of Parthenium dermatitis, both old and new, in the age
group 25-65 years, visiting the department on out-patient and in-patient
basis, confirmed by standard patch testing to Parthenium, were included
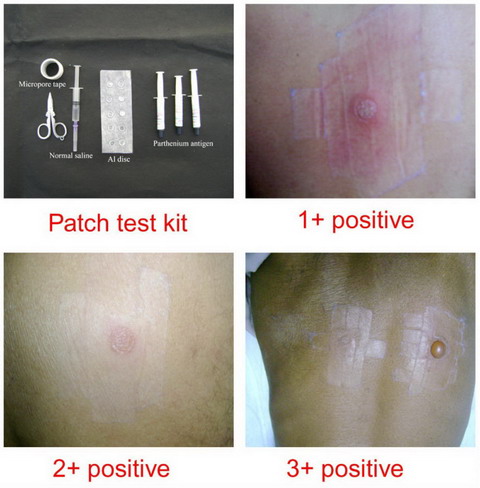
in the study after a proper written consent. Patch testing was done
using standard Parthenium patch test kit developed in R and D section of
Systopic labs New Delhi, through Contact and Occupational Dermatoses
Forum of India (CODFI), from which the kit was purchased. Each antigen
bottle contained 2 ml of 15% Parthenium antigen. It was ensured that
patients are not on local or systemic steroids for at least 2 weeks
prior to patch testing. Patch test result was read after 48 hours and
grading done according to International Contact Dermatitis Research
(ICDR) group as given in table 1 [32] (fig 2).
|
- |
Negative |
|
?+ |
Doubtful reaction ; faint erythema only |
|
+ |
Weak positive reaction ; palpable erythema, infiltration,
possibly papules |
|
++ |
Strong positive reaction; erythema, infiltration, papules,
vesicles |
|
+++ |
Extreme positive reaction ; intense erythema & infiltration,
coalescing vesicles |
|
IR |
Irritant reaction of different types |
Table 1: Grading of patch test
Patients <25 years or >65 years, pregnant and lactating females,
those with hypertension, diabetes, history of tuberculosis, liver or
kidney disease were not included in the study. The patients were
randomly divided into 2 equal groups of 30 patients each, statistically
comparable for each variable studied.
A detailed history was taken particularly regarding the grade of
itching, duration of present episode, total duration of disease,
seasonal variation and previous treatments taken. A general physical and
systemic examination was performed to clinically rule out any systemic
abnormality. Cutaneous examination with special reference to the
morphology, distribution, symmetry of lesions was performed and the
lesions were mapped on a chart. Pattern of Parthenium dermatitis was
noted. Clinical severity was assessed using the clinical severity score
(CSS), on the basis of itching, type of lesions and the area of body
involved, using the scoring method used in a previous study [33],
where 3 points each are given to itching and morphology and 4 points to
area of body involved, as follows:
a) Itching: 0 - no itching; 1 - mild; 2 - moderate; 3 - severe
itching
b) Morphology of lesions: 0 - no lesions; 1 - papules; 2 - plaques; 3
- lichenified plaques.
c) Area of body: 1 - face only; 2 - face, neck and hands; 3 - all
exposed sites and flexures; 4 - erythroderma.
Total score (a + b + c) is multiplied by 10 to get CSS of 100.
Each patient was subjected to baseline investigations like complete
haemogram, liver function tests, kidney function tests, urine analysis,
ECG, chest X ray at the start and at each follow up visit.
Thirty patients of the first group were given oral Azathioprine 300
mg weekly as a single dose of 6 tablets of 50 mg each, half an hour
after meals. Forty- eight hours before giving this large dose, an
initial test dose of 50 mg orally was given to look for any idiosyncrasy
and gastric intolerance.
Thirty patients of the second group were given oral Prednisolone 100
mg weekly in 2 divided doses of 50 mg each on 2 consecutive days.
Drugs were supplied free of cost to the patients. Therapy in both
groups was given for a total of 12 weeks. No topical corticosteroid or
other immunosuppressive agents were given during the study period other
than emollients and anti-histamines. Each patient was followed up at 4,
8 and 12 weeks. At each follow up, compliance to treatment was checked,
improvement in subjective symptoms noted and condition of cutaneous
lesions recorded. Improvement in cutaneous lesions of >80 % was taken as
marked, 60-80% as moderate and <60% as mild. Clinical severity score was
re-assessed, side effects and any abnormality in investigations after
the start of treatment noted.
At the end of study, the data was subjected to statistical analysis
performed by using computer software Microsoft Excel and SPSS 12.0 for
windows. Data was reported as percentage for qualitative variables and
CSS was reported as mean ± SD. Statistical significance and difference
between the two groups was evaluated using unpaired student `t` test and
chi square tests. A `P` value of <0.05 was considered statistically
significant. All `P` values used were two tailed. The efficacy was
assessed on the basis of a decrease in the mean CSS at 4, 8 and 12 weeks
follow up relative to the pre treatment clinical severity score (CSS 0).
Results
The demographic profile and other variables between the two study
groups are given in table 2 and 3.
|
Age (mean ± SD) |
46.8 ± 9.44 |
46.6 ± 8.35 |
T 0.10, p 0 .92 NS |
|
Sex (M/F) |
41084 |
41173 |
χ2 0.80 p 0 .37 NS |
|
Residence (R/U/SU) |
39615 |
39191 |
χ2 0.72 p 0.69 NS |
|
Occupation (Fa/S/O) |
39034 |
40402 |
Χ2 1.51 p 0.46 NS |
|
Grade of itching (Mild/Mod/Severe) |
1/29/0 |
1/24/5 |
Χ2 5.47 p 0.06 NS |
|
Duration of present illness in weeks (mean ±
SD) |
4.69 ± 3.75 |
3.68 ± 2 |
T 1.28 p 0.21 NS |
|
Duration of disease in years (mean ±
SD) |
6.33 ± 6.45 |
6.63 ± 5.43 |
T 0.20 p 0.84 NS |
|
CSS 0 (mean ± SD) |
66 ± 12.48 |
68.3 ± 10.85 |
T 0.77 p 0.44 NS |
|
Patch test positivity (1+/2+/3+) |
6/15/9 |
8/16/6 |
Χ2 0.92 p 0.63 NS |
Table 2: Comparison of various variables between the two study
groups
Note: M, Male; F, Female; Fa, Farmer; S, Service (Govt or Private);
O, Other (household or business); R, Rural; U, Urban; SU, Suburban; CSS
0, Clinical severity score at 0 week; NS, not significant
|
Age groups |
Azathioprine group (n=30) |
Prednisolone group (n=30) |
|
25 – 35 |
4 (13.33%) |
2 (6.67%) |
|
36 – 45 |
7 (23.33%) |
11 (36.67%) |
|
46 – 55 |
15 (50%) |
10 (33.33%) |
|
56 – 65 |
4 (13.33%) |
7 (23.33%) |
|
Total no of patients |
30 |
30 |
|
Mean age |
46.8 ± 9.44 |
46.6 ± 8.35 |
|
t 0.10 p 0 .92 NS |
Table 3: Distribution of patients as per age groups in
Azathioprine and Prednisolone groups
The pattern of Parthenium dermatitis in the two groups is given in table 4.
|
|
Azathioprine group (n = 30) |
Prednisolone
group (n = 30) |
|
Pattern of
disease |
Males |
Females |
Total |
Males |
Females |
Total |
|
Airborne
pattern |
12.000 |
5.000 |
17.000 |
15.000 |
6.000 |
21.000 |
|
-0.400 |
-0.167 |
-0.567 |
-0.500 |
-0.200 |
-0.700 |
|
Photo-exposed |
09 (30%) |
0.000 |
09 (30%) |
05(16.67%) |
03(10%) |
08 (26.67%) |
|
Erythroderma |
03(10%) |
0.000 |
03 (10%) |
0.000 |
0.000 |
0.000 |
|
Flexural |
0.000 |
01 (3.33%) |
01(3.33%) |
01(3.33%) |
0.000 |
01(3.33%) |
|
Total |
24(80%) |
06 (20%) |
30(100%) |
21(70%) |
09(30%) |
30(100%) |
Table 4: Distribution of patients as per pattern of Parthenium
dermatitis
The mean CSS at 0 week in the present study was 66 ± 12.48 in the
Azathioprine group and 68.3 ± 10.85 in the Prednisolone group, with no
statistically significant difference in pre treatment score between the
two groups (t 0.77 p 0.44 NS).
At 4 weeks, in the Azathioprine group, the mean CSS decreased to a
value of 51.7 from the mean value of 66 at 0 week, with statistically
significant percentage decline in mean of 47.78 (t 3.97 p 0.0002 S) and
in the Prednisolone group, the mean CSS at week 4 decreased from 68.3 at
0 week to a mean CSS value of 63 at week 4 with statistically
insignificant percentage decline of 17.78 (t 1.82 p 0.0735 NS).
At 8 weeks, in the Azathioprine group, the mean CSS decreased to a
value of 39 from the mean value of 66 at 0 week, with a percentage
decline of 90, with high statistical significance (t 7.15 p 0.0001 S)
and in the Prednisolone group, the mean CSS decreased to a level of 52.3
from the mean value of 68.3 at 0 week, with statistically significant
percentage decline of 53.3 (t 5.23 p 0.0001 S).
At 12 weeks, in the Azathioprine group, the mean CSS decreased to a
value of 22.3 from the mean value of 66 at 0 week, with statistically
significant percentage mean decrease of 145.55 (t 12.03 p 0.0001 S) and
in the Prednisolone group, the mean CSS at 12 weeks decreased from 68.3
at 0 weeks to a mean value of 43.3 at 12 weeks with statistically
significant percentage decrease of 83.33 (t 6.21 p 0.0001 S) (table
5, 6 and figs 3, 4, 5, 6, 7).
|
CSS (mean ± SD) |
Azathioprine
group (n=30) |
Prednisolone
group (n=30) |
Significance |
|
0 weeks |
66 ±12.48 |
68.3 ±10.85 |
T 0.77 p 0.44 NS |
|
4 weeks |
51.7 ±15.33 |
63 ±11.79 |
T 3.21 p 0.0022 S |
|
8 weeks |
39 ±16.47 |
52.3 ±12.78 |
T 3.50 p 0.0009 S |
|
12 weeks |
22.3 ±15.46 |
43.3 ±19.18 |
T 4.67 p 0.0001 S |
Table 5: Inter group comparison of efficacy of Azathioprine
versus Prednisolone at 4, 8, 12 weeks
|
|
Azathioprine group |
Prednisolone group |
|
|
0 weeks |
4 weeks |
8 weeks |
12 weeks |
0 weeks |
4 weeks |
8 weeks |
12 weeks |
|
Sample size |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
|
CSS |
66± |
51.7± |
39± |
22.3± |
68.3± |
63± |
52.3± |
43.3± |
|
(mean± SD) |
12.48 |
15.33 |
16.47 |
15.46 |
10.85 |
11.79 |
12.78 |
19.18 |
|
Signifi-cance |
|
t3.97p .0002 |
t7.15p .0001 |
t12.0p 0.0001 |
|
t1.82p .0735 |
T5.23p .0001 |
t 6.21p 0.0001 |
|
Mean % difference |
|
47.78 |
90 |
145.55 |
|
17.78 |
53.33 |
83.33 |
Table 6: Intra group comparison of efficacy of Azathioprine
versus Prednisolone at 4, 8, 12 weeks to that at 0 week
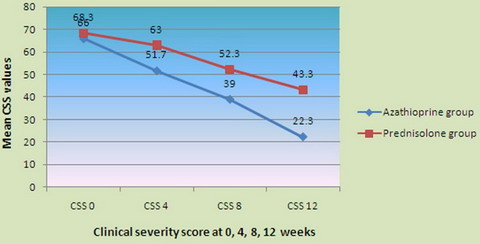
| Fig
3: Comparison of efficacy at 0, 4, 8, 12 weeks. |

| Fig
4: Azathioprine Group (patient 1). |
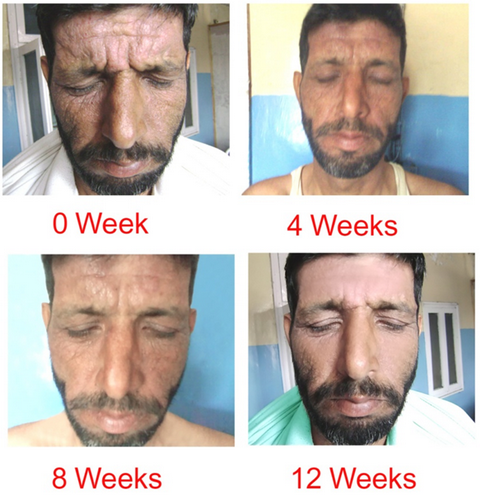
| Fig
5: Azathioprine Group (patient 2). |

| Fig
6: Prednisolone Group (patient 1). |
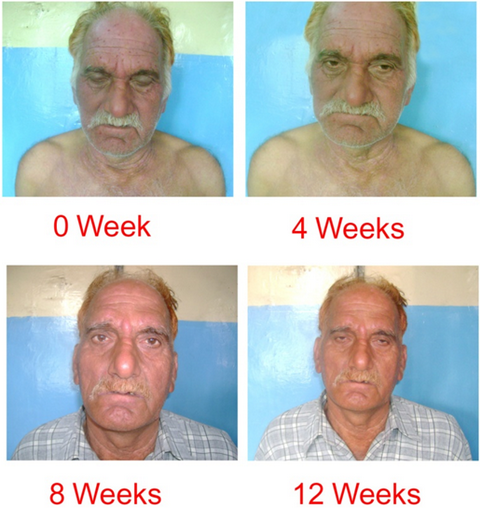
| Fig
7: Prednisolone Group (patient 2). |
The side effect profile detected in the two patient groups is shown
in table 7.
|
Side effects (clinical, investigational) |
Azathioprine group (n=30) |
Prednisolone group (n=30) |
|
Abnormality in Hb, TLC, Platelet count |
0 |
0 |
|
Abnormal LFT |
|
02 (6.67%) |
|
Abnormal B sugar |
|
02 (6.67%) |
|
Abnormal KFT |
0 |
0 |
|
Abnormality in ECG, chest X ray |
0 |
0 |
|
Diarrhea |
04 (13.33%) |
01 (3.33%) |
|
Nausea, Vomiting |
02 (6.67%) |
04 (13.33%) |
|
Epigastric pain |
03 (10%) |
06 (20%) |
|
Folliculitis |
01 (3.33%) |
|
|
Headache/ drowsiness |
01 (3.33%) |
03 (10%) |
|
Others (dry mouth, aches/pains) |
01 (3.33%) |
01 (3.33%) |
|
*Few patients developed more than one side effect in the
same patient |
Table 7: Side effect profile in both Azathioprine and
Prednisolone groups
Discussion
The present study included 60 patients in the age group 25-65 years,
with mean age of 46.7 years, consistent with previous studies [27,29,30,31,34].
Forty- three patients out of 60 were in the age group 36-55 years,
probably due to higher chances of exposure to Parthenium weed because of
occupational or recreational activities.
There were 45 males and 15 females, with a sex ratio of 3: 1,
consistent with that in other studies [22,27,30,31,34,35,36].
The increased prevalence of Parthenium dermatitis in males may be
because of more exposure to parthenium [5].
Out of the 60 patients, 35 (58.33%) came from rural areas, 15 (25%)
from suburban and 10 (16.66%) from urban background. No previous study
laid emphasis on the residential background of patients. We included it
to ensure comparability in the two study groups and that people from
rural and suburban areas may be more exposed to Parthenium weed than
urban lot.
Twenty- five (41.66%) patients were farmers and involved with working
in the field. Nineteen (31.66%) patients belonged to service class but
frequently exposed directly to plant. Sixteen (26.66%) patients, were
either housewives, elderly persons or business men. This shows more
prevalence of disease with direct exposure to the plant than with
indirect contact [5,37].
Various variables like level of itching, duration of present illness,
grading of patch test positivity was recorded to make the two groups
comparable and to remove confounding factors as well as to look for
their effect on the clinical presentation of the disease.
Parthenium dermatitis is a chronic relapsing and remitting disease.
The mean duration of the disease in years in the Azathioprine and the
Prednisolone groups was 6.33 ± 6.45 and 6.63 ± 5.43 respectively,
consistent with other studies [27,29,30,31,36].
Parthenium dermatitis presents in various patterns like airborne contact
dermatitis, dermatitis of hands and face, chronic actinic dermatitis,
photo-dermatitis, disseminated forms, hand dermatitis, prurigo nodularis,
photosensitive lichenoid eruption, erythroderma, seborrheic and many
other patterns [5,35,38,39,40,41,42].
Air borne contact dermatitis is the most common pattern [5,18,29,36],
as found in the present study, 38 (63.33%) out of 60 patients.
The mainstay of treatment for parthenium dermatitis has been with the
use of topical and systemic steroids. The use of steroids is associated
with several serious and often irreversible side effects [13,14],
observed more with daily regimens than pulse therapy regimens [17].
Various immunosuppressive agents are a good alternative to steroids for
use in Parthenium dermatitis. Azathioprine is a potent immunosuppressant
and its immunosuppressive action results from inhibition of purine
synthesis, thus blocking DNA replication in T-cells and Langerhan cells
[43] and suppressing cell mediated
immune reactions [44]. Azathioprine is
converted into its inactive metabolites mainly by Thio Purine Methyl
Transferase (TPMT). The absence or deficiency of this enzyme, seen in
0.5% of normal population who are homozygotes for low activity allele
may lead to Azathioprine toxicity [45].
So, prior estimation of TPMT enzyme levels is necessary before starting
therapy with Azathioprine. The preliminary studies on the use of
Azathioprine in air borne contact dermatitis, actinic reticuloid,
chronic actinic dermatitis and also in atopic dermatitis have given
encouraging results [20,21,22,23,24]
and it is inferred that Azathioprine is an effective corticosteroid
sparing agent with lesser side effect profile for use in Parthenium
dermatitis [24,25,26,27,29,30,46].
The therapeutic response to Azathioprine usually becomes appreciable
after few weeks [29,47],
in contrast to earlier and rapid response in the present study.
In the Azathioprine group of patients, the mean CSS at 4, 8 and 12
weeks decreased respectively to values of 51.7, 39 and 22.3 from the
mean value of 66 at 0 week, with statistically significant percentage
decline in the mean of 47.78 (t 3.97 p 0.0002 S), 90 (t 7.15 p 0.0001 S)
and 145.55 (t 12.03 p 0.0001 S) respectively. Such high efficacy of
Azathioprine is consistent with other studies [31,27,29,46].
Pulse therapy of steroids, where supra pharmacological doses of steroids
are given over a short period of time, has also promised faster response
and stronger efficacy with decreased major side effect profile than the
use of long term daily regimens of steroid therapy [17].
Pulse therapy may be administered either by intravenous route or orally,
called oral mini pulse therapy. Oral mini pulse of steroids has been
used effectively in many dermatological conditions like alopecia areata
[48,49,50],
connective tissue diseases [17], lichen
planus [18,22,27],
pemphigus vulgaris [53], vitiligo [54]
and is also promising for Parthenium dermatitis.
In the present study, in the Prednisolone group of patients, the mean
CSS at 4, 8 and 12 weeks decreased from 68.3 at 0 week to mean CSS
values of 63, 52.3 and 43.3 respectively, with percentage decline of
17.78 (t 1.82 p 0.0735 NS), 53.3 (t 5.23 p 0.0001 S) and 83.33 (t 6.21 p
0.0001 S) respectively. Thus patients on oral mini pulse of Prednisolone
also showed significant response, though the response was statistically
much more significant in the Azathioprine group than that in the
Prednisolone group at each follow up (t 3.21 p 0.0022 S, t 3.50 p 0.0009
S, t 4.67 p 0.0001 HS) respectively at 4, 8 and 12 weeks, as found in
previous studies [30].
Total therapy in the present study was given for a period of 12
weeks, in contrast to previous studies where treatment was given for a
longer duration.
In the present study, it was found that there was a progressive
decrease in the mean CSS with Azathioprine at each successive follow up
(table 6), consistent with previous studies [27,29,31].
Thus, the longer the therapy is given, the better the efficacy, with
progressive decrease in severity score. Oral mini pulse with
Prednisolone also showed increasingly good response at each follow up.
Though there was excellent response to pulse therapy with both the
drugs, but relapse rates were higher after stopping the treatment at 12
weeks and this was detected in those patients who were followed up for a
longer period after stopping the treatment at 12 weeks.
Long-term use of Azathioprine is associated with various side effects
like nausea, vomiting, abdominal pain, fever, hepatitis, transient rise
in liver enzymes, bone marrow suppression, renal toxicity, diffuse
alopecia, various infections, acneiform eruptions, oral ulcers,
pigmentation of nails, various premalignant and malignant neoplasms and
rarely shock [34,43,55,56,57,58,59,60,61].
Side effects are likely to increase in patients with deficient or absent
Thiopurine Methyl Transferase (TPMT) enzyme levels. In the present
study, prior estimation of TPMT levels was not done due to lack of
facilities. We detected few minor side effects with Azathioprine weekly
pulse (table 7), consistent with previous studies [29,34].
Studies have shown decreased side effects of oral mini pulse therapy of
steroids than the daily regimens [17],
consistent with few side effects in the present study (table 7).
Thus, Azathioprine is an effective and relatively safe alternative to
systemic steroids in the treatment of Parthenium dermatitis and use of
weekly pulse therapy can further increase the compliance and safety and
reduce the cost of therapy. Patients on Azathioprine should be regularly
monitored to detect serious adverse effects at an earliest, particularly
after prolonged use of the drug. Estimation of Thiopurine Methyl
Transferase (TPMT) enzyme levels should be done before the start of
treatment, wherever such facilities exist. Oral mini pulse therapy with
steroids can also be used as an effective alternative to daily systemic
steroids, with decreased side effects than the later. There were few
lacunae in the present study which need to be addressed in subsequent
studies, like less number of patients in the two study groups, less
duration of treatment and short follow up of 12 weeks only.
References
1. Aneja KR, Dhawan SR, Sharma AB. Deadly weed
Parthenium hysterophorus L. and its distribution. Indian J Weed Sci
1991; 23: 14-8.
2. Lonkar A and Jog MK. Dermatitis caused by a plant
Parthenium Hysterophorus Linn. A preliminary report. Indian J dermatol
Venereol leprol 1968; 34:194-196.
3. Mitchell JC. Calnan CD. Scourge of India: Parthenium
dermatitis. Int J Dermatol 1978; 17: 303-4.
4. Lakshmi C, Srinivas CR. Parthenium: A wide angle
view. Indian J Dermatol Venereol Leprol 2007; 73: 296-306.
5. Sharma SC and Kaur S. Contact dermatitis from
Composite plants. Indian J Dermatol Venereol Leprol1990; 56: 27-30.
6. Sayal SK, Das L, Kumar A. Study of clinical profile
of allergic contact dermatitis in Pune. Indian Journal of Dermatology,
1999; 44 (3).
7. Tiwari VD, Sohi AS and Chopra TR. Allergic contact
dermatitis due to Parthenium hysterophorus. Indian J Dermatol Venereol
Leprol 1979 ; 45: 392-400.
8. Pasricha JS, Nandakishore Th. Air-borne contact
dermatitis due to Chrysanthemum with true cross sensitivity to
Parthenium hysterophorus and Xanthium strumarium. Indian J Dermatol
Venereol Leprol 1992; 58: 268-71.
9. Nandakishore T and Pasricha JS. Pattern of cross
sensitivity between four Compositae plants, Parthenium hysterophorus,
Xanthium strumarium, Halianthus annuus and Chrysanthemum coronarium, in
Indian patients. Contact Dermatitis 1994 Mar; 30(3): 162-7.
10. Srinivas CR, Krupashankar DS and Singh KK. Oral
hyposensitization in Parthenium dermatitis. Contact Dermatitis 1998; 18:
242.
11. Bose KS. Pentoxiphylline in contact
hypersensitivity reactions. Indian J Dermatol Venereol Leprol 1996; 62:
135-7.
12. Dogra S, Kanwar AJ. Narrow band UVB phototherapy in
dermatology. Indian J Dermatol Venereol Leprol 2004; 70: 205-9.
13. Storrs FJ. Use and abuse of systemic corticosteroid
therapy. J Am Acad Dermatol 1979; 1: 95-105.
14. Gallant C and Kenny P. Oral Glucocorticoids and
their complications. A review. J Am Acad Dermatol 1986; 14: 161-77.
15. Truhan AP and Ahmed AR. Corticosteroids: a review
with emphasis on complications of prolonged systemic therapy. Ann
Allergy 1989; 62: 375-90.
16. Lester RS, Knowles SR and Shear NH. The risks of
systemic corticosteroid use. Dermatol Clin 1998; 16: 277-88.
17. Dogra A, Monika J, Gurkirat SB, Anju Ag. A
comparative study of ocular side effects of pulse steroid therapy versus
long term oral steroid therapy in steroid responsiveness dermatoses.
International Journal of Leprosy and Other Mycobacterial Diseases, Sep
2002.
18. Sharma VK, Bhat R, Sethuraman G, Manchanda Y.
Treatment of Parthenium dermatitis with Methotrexate. Contact dermatitis
2007-Aug; 57 (2): 118-9.
19. Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in
Parthenium dermatitis -a report of 2 cases. Contact Dermatitis 2008 Oct;
59 (4): 245-8.
20. Roed PJ and Thomsen K. Azathioprine in the
treatment of air borne contact dermatitis from Compositae oleoresins and
sensitivity to UVA. Acta Dermatol Venereol (Stockholm) 1980; 60:
275-277.
21. August PJ. Azathioprine in the treatment of eczma
and actinic reticuloid. Br J Dermatol 1982; 107 (suppl): 22-23.
22. Leigh IM and Hawk JLM. Treatment of chronic actinic
dermatitis with Azathioprine. Br J Dermatol 1984; 110: 691-695.
23. Meggitt SJ and Reynolds NJ. Azathioprine for atopic
dermatitis. Clin Exp Dermatol 2001; 26: 369-75.
24. Verma KK, Mittal R. Azathioprine as a therapeutic
modality for the treatment of severe adult atopic dermatitis. Indian J
Dermatol Venereol Leprol 2001; 67: 189-91.
25. Srinivas CR, Balachandran C, Shenoi SD and Acharya
S. Azathioprine in the treatment of Parthenium dermatitis. Br J Dermatol
1991; 124: 394-5.
26. Verma KK and Pasricha JS. Azathioprine as a
corticosteroid sparing agent in air borne contact dermatitis. Indian J
Dermatol Venereol Leprol 1996; 62: 30-2.
27. Sharma VK, Chakrabarti A and Mahajan V.
Azathioprine in the treatment of Parthenium dermatitis. Int J Dermatol
1998; 37: 299-302.
28. Verma KK, Manchanda Y and Pasricha JS. Azathioprine
as corticosteroid sparing agent for the treatment of dermatitis caused
by the weed Parthenium. Acta Dermatol Venereol 2000; 80: 31-2.
29. Verma KK, Bansal A and Sethuraman G. Parthenium
dermatitis treated with Azathioprine weekly pulse doses. Indin J
dermatol Venereol Leprol 2006; 72: 24-27.
30. Verma KK, Mahesh R, Srivastava P, Ramam M,
Mukhopadhyaya AK. Azathioprine versus Betamethasone for the treatment of
Parthenium dermatitis: A randomized controlled study. Indian J Dermatol
Venereol Leprol 2008; 74: 453-7.
31. Khurana S, Minocha YC, Minocha KB and Dogra A.
Evaluation on Azathioprine in the treatment of Parthenium dermatitis.
Indian J Dermatol Venereol Leprol 2001; 67: 309-311.
32. Wilkinson DS, Fregert S, Magnusson B et al.
Terminology of contact dermatitis. Acta Derm Venereol (Stockh) 1970; 50:
287-92.
33. Verma KK, Manchanda Y, Dwivedi SN. Failure of titer
of contact hypersensitivity to correlate with clinical severity and
therapeutic response in contact dermatitis caused by Parthenium. Indian
J Dermatol Venereol Leprol 2004; 70: 210-13.
34. Kaushal K and Manchanda Y. Long term safety and
toxicity of Azathioprine in patients with airborne contact dermatitis.
Indian J Dermatol Venereol Leprol 2001; 67: 75-7.
35. Verma KK, Sirka CS, Ramam M and Sharma VK.
Parthenium dermatitis presenting as photosensitive lichenoid eruption: a
new clinical variant. Contact Dermatitis 2002; 46: 286-9.
36. Sharma VK, Sethuraman G, Bhat R. Evolution of
clinical pattern of Parthenium dermatitis: a study of 74 cases. Contact
Dermatitis. 2005 Aug; 53(2): 84-8.
37. Lonkar A, Mitchell JC and Calnan CD. Allergic
contact dermatitis from Parthenium hysterophorus. Trans St John Hosp
Dermatol Soc 1974; 60: 43-49.
38. Bhutani LK and Rao DS. Photocontact dermatitis
caused by Parthenium hysterophorus. Dermtatologica 1978; 157: 206-9.
39. Lynette A Gordon. Compositae Dermatitis. Australas
J Dermatol 1999; 40(3): 123-130.
40. Sharma V K, Sahoo B. Prurigo nodularis like lesion
in Parthenium dermatitis. Contact Dermatitis. 2000 Apr; 42(4): 235.
41. Agarwal, Kumar Nath, Jaisankar, Dsouza. Parthenium
dermatitis presenting as erythroderma. Contact Dermatitis 2008; 59 (3):
182-183.
42. Sethuraman G, Bansal A, Sharma VK, Verma KK.
Seborrheic pattern of Parthenium dermatitis. Contact Dermatitis. 2008
Jun; 58(6): 372-4.
43. Ahmed AR and Mox R. Azathioprine. Int J Dermatol
1981; 20: 461-7.
44. Pasricha JS. Contact Dermatitis in India, 2nd edn,
Offset publishers, New Delhi, Department of Science and Technology,
1998.
45. Snow JL, Gibson LE. The role of genetic variation
in Thiopurine methyl transferase activity and the efficacy and/ or side
effects of Azathioprine therapy in dermatologic patients. Arch Dermatol
1995; 131: 193-7.
46. Verma KK, Mittal R, Manchanda Y and Khaitan BK.
Lichen planus treated with Betamethasone oral minipulse therapy. Indian
J Dermatol Venereol Leprol 2000; 66: 34-35.
47. Breathnach S.M, Griffiths C.E.M, Chalmers R.J.G &
Hay R.J. Syatemic therapy. In: Tony Burns, Stephen Breathnach, Neil Cox,
Christopher Griffiths, editors. Rook`s text book of Dermatology. 7th ed.
Blackwell Sciences; 2004. p. 72.23.
48. Pasricha JS, Kumrah L. Alopecia totalis treated
with oral mini-pulse (OMP) therapy with Betamethasone. Indian J Dermatol
Venereol Leprol 1996; 62: 106-9.
49. Khaitan BK, Mittal R, Verma KK. Extensive alopecia
areata treated with Betamethasone oral mini-pulse therapy: An open
uncontrolled study. Indian J Dermatol Venereol Leprol 2004; 70: 350-3.
50. Kar BR, Handa S and Dogra S. Placebo controlled
oral pulse therapy in alopecia areata. J Am Acad dermatol 2005; 52:
287-290.
51. Ramesh M, Balachandran C, Shenoi SD and Rai VM.
Efficacy of steroid oral mini-pulse therapy in lichen planus: An open
trial in 35 patients. Indian J Dermatol Venereol Leprol 2006; 72:
156-157.
52. Kelett JK and Ead RD. Treatment of lichen planus
with a short course of oral Prednisolone. Br J Dermatol 1990; 123:
550-551.
53. Pasricha JS, Khaitan BK, Raman RS and Chandra M.
Dexamethasone Cyclophosphamide pulse therapy for pemphigus. Int J
Dermatol 1995; 34: 875-82.
54. Rath N, Kar HK, Sabhnani S. An open labeled,
comparative clinical study on efficacy and tolerability of oral
minipulse of steroid (OMP) alone, OMP with PUVA and broad/ narrow band
UVB phototherapy in progressive vitiligo. Indian J Dermatol Venereol
Leprol 2008; 74: 357-60.
55. Sturdevant RAL, Singleton JW, Deren JJ et al.
Azathioprine related pancreatitis in patients with Crohn`s disease.
Gastroenterology 1979; 77: 883-6.
56. Phillips T, Salisbury J, Leigh I and Barker H. Non
Hodgkin`s Lymphoma associated with long term Azathioprine therapy. Clin
Exp Dermatol 1987; 12: 444-5.
57. Silman A, Petrie J, Hazleman B and Evans S.
Lympho-proliferative cancer and other malignancies in patients with
rheumatoid arthritis treated with Azathioprine: A 20 year follow up
study. Ann Rheum Dis 1988; 47: 988-92.
58. Jones JJ, Ashworth J. Azathioprine induced shock in
dermatology patients. J Am Acad Dermatol 1993; 29: 795-6.
59. Connell WR, Kamm MA and Nixon M. Long term
neoplasia risk after Azathioprine treatment in inflammatory bowel
disease. Lancet 1994; 343: 1249-52.
60. Bottomley WW, Ford G and Cunliffe WJ. Aggressive
squamous cell carcinomas developing in patients receiving long term
Azathioprine. Br J Dermatol 1995; 133: 460-462.
61. Pise GA, Vetrichevvel TP, Thappa DM.
Azathioprine-induced shock: An uncommon, unpredictable and potentially
fatal adverse effect of Azathioprine. Indian J Pharmacol 2007; 39:
115-6.
© 2012 Egyptian Dermatology
Online Journal
|