|
|
Abstract
Background : Alopecia areata (AA) is a non-scarring, inflammatory,
hair loss disease with an unpredictable course. It is commonly manifested
by patchy areas of complete hair loss on the scalp and other hair-bearing
areas in the body that can progress to complete loss of all body hair. AA
is a T-cell-mediated autoimmune condition that is most likely to occur in
genetically predisposed individuals. Different cytokines such as Interleukin
1 ( IL-1), tumor necrosis factor alpha (TNF-α)
and Interferon gamma (IFN-γ)
are thought to be involved in the pathogenesis of AA. Proper cell metabolism
and effective immune function are dependent on a total of 72 trace elements
e.g., zinc (Zn), copper (Cu) and selenium (Se). Disturbances in the levels
of Zn, Cu and Se are thought to play a role in the pathogenesis of AA through
affecting the levels of different cytokines.
Aim of the Work: The aim of this study was to explore the
role could be played by the trace elements ( Zn, Cu and Se ) in the pathogenesis
of AA.
Patients and Methods: This study included 44 patients with
a single patch of AA (group I), 36 patients with multiple patches of AA
(group II) and 20 healthy control individuals (group III). Venous blood
samples were taken from all the participants and then these samples were
analyzed by flame atomic absorption spectrophotometer (FAAS) to estimate
the serum level of Zn, Cu and Se.
Results: There were statistically significant decrease
in the serum level of Zn, Cu and Se of both groups of AA patients than controls,
but there was no difference in the serum levels of the studied elements
between the two groups of AA patients. There was a statistically significant
decrease in serum level of Zn and Cu in males of group II than those of
group I, also, there was a statistically significant decrease in serum level
of Se in the females of group II than group I. There was a statistically
significant difference in the serum level of Zn in group I and II in age
≤
30 and > 30 years. There was a statistically significant decrease
in serum level of Zn (group II) than (group I) when the duration of the
disease is
≤
2 months. There were no significant relations between the studied trace
elements and the rest of clinical parameters.
Conclusion: Serum of both groups of AA patients expressed
low levels of Zn, Cu and Se when compared with controls suggesting their
possible role in the pathogenesis of AA.
Introduction
Alopecia areata is a chronic, non scaring inflammatory disease which
affects the hair follicles (HF). The onset may be at any age and there is
no known race or gender predominance.[1]
AA can present with many different patterns; a flat alopecic patch with
normal skin color, involving the scalp or any other pillar region of the
body is the characteristic lesion of AA but the disease can progress to
complete loss of all body hair.[2] AA may
be associated with nails abnormalities; as pitting and longitudinal or transverse
striations. Sometimes, concurrent atopic disorders, diabetes mellitus and
other autoimmune disorders may alter the course and prognosis.[3]
The patho-physiology of AA is considered to be T-cell mediated autoimmunity
that occurs mostly in genetically predisposed individuals.[4]
In addition to the disturbance of the immune function, complex interactions
between predisposing genetic and environmental factors act as triggers for
disease progression.[5]
In order to maintain vital processes, trace elements (e.g. Zn, Cu and
Se) must be present in the human body within certain concentration ranges.
They fulfill special biological functions e.g. as catalysts in the synthesis
of proteins and enzymes.[6] Zn is one of
the most abundant nutritionally essential elements in the human body. It
has both catalytic and structural roles in enzymes.[7]
Cu is an essential trace element, important for the function of many cellular
enzymes.[8] It is the third most abundant
trace element found in the body (after iron and Zn).[9]
Se, an essential trace element that has a protective, immune modulating
and anti-proliferative properties.[10]
Many of the functions of Se are thought to be mediated by selenoproteins
that contain Se in the form of the amino acid, selenocysteine.[11]
There are claims that imbalance of trace elements in the serum of AA patients
may trigger the onset of the disease.[12]
The aim of this work was to evaluate and provide an insight about the
possible role of the trace elements, ( Zn, Cu and Se ) in the etiopathogenesis
of AA.
Patients And Methods
Patients
The current study comprised a total of 80 patients of different varieties
of AA (single and multiple patches) in addition to 20 healthy individuals
who were randomly selected as a control group with no history of any systemic
or skin disease. All persons, representing both genders and different age
groups. The patients were diagnosed on the basis of the typical appearance
of the skin lesions. The studied persons were collected from the Outpatient
Clinic of Dermatology and Venereology Department, Tanta University Hospitals
during the period from March to September 2011.
The studied persons were divided into the following groups:
Group I : included 44 patients with single patch of AA.
Group II : included 36 patients with multiple patches of AA.
Group III : included the 20 healthy persons served as a control
group.
Inclusion criteria
Newly diagnosed cases who did not receive any topical or systemic treatment
in the past 6 weeks and patients who did not have any other skin (e.g. atopic
dermatitis, lichen planus or vitiligo) or systemic diseases (e.g. thyroid
disease, collagen-vascular diseases, diabetes mellitus, down syndrome, autoimmune
polyendocrine syndrome type I, myasthenia gravis, pernicious anemia or ulcerative
colitis). All patients agreed to join the study and signed a written consent.
Exclusion criteria
Old AA cases who received recent systemic treatment in the past 6 weeks,
pregnant and lactating females or those who were receiving any hormonal
contraceptive drugs and patients currently taking nutritional supplements,
magnesium containing laxatives or diuretics.
All persons in this study were subjected to the following
Full history taking including personal history, present history, past
history of diseases or drugs used and family history, thorough general examination,
detailed dermatological examination including; skin, mucous membrane and
nail examination and laboratory investigations which include: routine laboratory
investigations, thyroid function tests and estimation of serum level of
Zn, Cu and Se by FAAS.
Methods
Collection and preparation of serum samples
Venous blood samples were withdrawn, from the antecubital vein, using
a dry wide pore needle in all cases. Blood was put in glass tubes previously
treated with deionized water and dried. The samples were then allowed to
clot in a water bath at 37°C and centrifuged at 3000 rpm for 5 minutes.
The collected serum was put into dry test tubes, covered and kept in the
refrigerator at - 20°C till analysis.
Determination of trace elements
The method used in this work was the flame technique of atomic absorption
spectro-photometer (FAAS) for determination of Zn, Cu and Se.
Flame atomic absorption spectrophotometer
It is a spectroanalytical procedure for the qualitative and quantitative
determination of chemical elements employing the absorption of optical radiation
(light) by free atoms in the gaseous state (Fig. 1). FAAS can be used to
determine over 70 different elements in a solution or directly in solid
samples. Since samples are usually liquids or solids, the analyte atoms
or ions must be vaporized in a flame or graphite furnace. The atoms absorb
ultraviolet or visible light and make transitions to higher electronic energy
levels. The analyte concentration is determined from the amount of absorption.
Concentration measurements are usually determined from a working curve after
calibrating the instrument with standards of known concentration.[13]
 | Fig 1:
Picture of a flame atomic-absorption spectrophotometer apparatus
(13) |
|
Statistical analysis
All data obtained were transferred to the statistical package for the
social sciences version 15 (IBM Co., New York, USA) for analysis. Data were
summarized using mean, standard deviation (mean± SD) and compared using
student's -t test. Comparison between groups were made by using X2 - test
and Fissure's exact test for quantitative variables Statistical significance
was determined at a level of p < 0.05*
Results
Clinical results
*The clinical photos of AA patients were illustrated in Fig. 2 and 3.
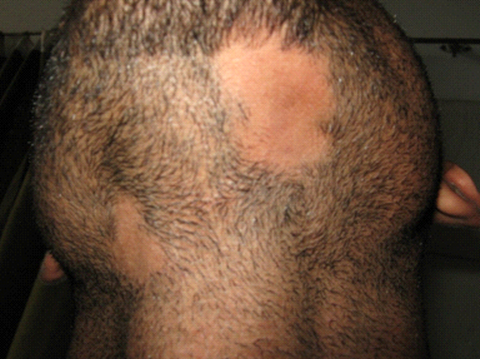 | Fig
2: Male patient with two patches of alopecia areata in the
beard area. |
|
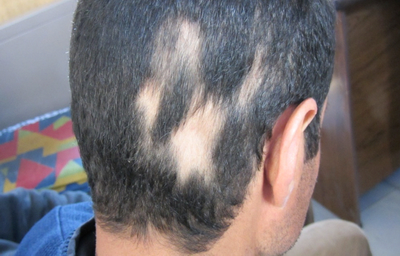 | Fig
3: Male patient with multiple patches of alopecia areata in the
scalp. |
|
* The clinical criteria of the studied groups were demonstrated in Table
1.
|
|
Groups |
Chi-square |
|
Group |
Group |
Group |
Total |
T- test |
X2 |
P-value |
|
I |
II |
III |
Age
/years |
|
N |
44 |
36 |
20 |
100 |
0.11 |
|
0.913 |
|
Range |
18-42 |
20-45 |
18-45 |
18-45 |
|
Mean±SD |
28±7.4 |
30.7±9.6 |
30.20± |
29.5 |
|
7.69 |
+ |
|
|
8.1 |
|
Gender |
Male |
N |
12 |
28 |
10 |
50 |
|
|
0.639 |
|
% |
20 |
46.66 |
33.33 |
100 |
|
|
Female |
N |
32 |
8 |
10 |
50 |
0.532 |
|
% |
53.3 |
13.3 |
33.33 |
100 |
|
|
Duration |
|
Mean±SD |
1.72±0.81 |
3.11 |
|
|
0.325 |
|
0.725 |
|
/months |
± 1.96 |
- |
|
|
|
|
|
Family history |
Negative |
N |
44 |
32 |
- |
76 |
|
|
- |
|
% |
100 |
88.9 |
- |
95 |
|
|
|
Positive |
N |
- |
4 |
- |
4 |
- |
|
|
% |
- |
11.1 |
- |
5 |
|
Significant P-value < 0.05
Table (1): Comparison between alopecia areata groups and controls
as regards age, gender, duration of the disease and family history
Laboratory results
* There was a statistically significant decrease in serum Zn, Cu and
Se level in AA groups than controls, but there was no significant difference
in serum Zn, Cu and Se level between group I and group II AA patients (Table 2).
|
|
Groups |
Chi-square |
Group I& II |
|
Group I |
Group II |
Group III |
t-test |
P-value |
t-test |
p. value |
|
Zinc |
Range |
0.3-0.76 |
0.1-0.86 |
0.66-1.87 |
4.235 |
0.021 |
0.801 |
0.711 |
|
(ppm) |
Mean±SD |
0.57±0.16 |
0.56±0.30 |
0.66±0.21 |
|
Copper |
Range |
0.49-1.24 |
0.46-1.34 |
1.12-1.42 |
3.253 |
0.042 |
0.751 |
0.608 |
|
(ppm) |
Mean±SD |
0.93±0.24 |
0.95±0.28 |
1.237± |
|
|
0.17 |
|
Selenium |
Range |
0.12-0.36 |
0.11-0.35 |
0.37-0.55 |
4.232 |
0.031 |
1.025 |
0.847 |
|
(ppm) |
Mean±SD |
0.22±0.09 |
0.25±0.09 |
0.431± |
|
|
0.058 |
Significant P-value < 0.05
Table (2): Serum level of zinc, copper, selenium in patients and
controls
* There was a statistically significant decrease in Zn/Cu ratio and increase
in Cu/Se ratio between AA groups and controls, but no significant difference
was detected between both AA groups (Table 3).
* There was a statistically significant difference in Zn/Se ratio between
both AA groups, but there was no significant difference between AA groups
and controls (Table 3).
|
|
Groups |
t-test |
P-value |
Group I & II |
|
Group I |
Group II |
|
t-test |
P-value |
|
Group III |
|
Zinc/copper |
Range |
0.06-0.65 |
0.05-0.61 |
0.54-1.50 |
3.624 |
0.015 |
|
|
|
Ratio |
Mean±SD |
0.418+0.17 |
0.395+0.16 |
0.794+0.27 |
1.253 |
0.06 |
|
Zinc/selenium ratio |
Range |
0.38-5.36 |
0.40-7.74 |
1.20-4.92 |
0.528 |
0.324 |
|
|
|
Mean±SD |
2.220+0.63 |
3.02+1.90 |
2.33+0.96 |
2.885 |
0.047 |
Copper
/selenium ratio |
Range |
2.11-15.33 |
2.90-16.10 |
2.18-3.64 |
2.663 |
0.047 |
|
|
| |
Mean±SD |
8.36+1.25 |
7.31+2.40 |
2.91+0.44 |
0.652 |
0.421 |
Significant P-value < 0.05
Table (3): Zinc/copper ratio, zinc/selenium ratio and copper/selenium
ratio in alopecia areata patients and controls
* As regards gender, there was a statistically significant decrease in
serum level of Zn and Cu in males of group II than those of group I and
there was a statistically significant decrease in serum level of Se in females
of group II than those of group I (Table 4).
|
|
Groups |
t-test |
P-value |
|
Group I |
Group II |
|
Zinc |
male |
0.610+0.210 |
0.512+0.066 |
4.582 |
0.036 |
|
(ppm) |
female |
0.550±0.201 |
0.511±0.152 |
1.585 |
0.096 |
|
Copper |
male |
1.30±0.301 |
1.14±0.201 |
2.529 |
0.044 |
|
(ppm) |
female |
1.61±0.330 |
1.550±0.34 |
0.324 |
0.429 |
|
Selenium |
male |
0.240±0.101 |
0.216±0.040 |
2.056 |
0.057 |
|
(ppm) |
female |
0.231±0.034 |
0.203±0.047 |
3.629 |
0.047 |
Significant P-value < 0.05
Table (4): Comparison between serum level of zinc, copper, selenium
and gender in alopecia areata groups
* As regards age, there was a statistically significant difference in
the serum level of Zn in group I and II in age
≤
30 and > 30 years (Table 5).
|
Age/ years |
Group I |
Group II |
t-test |
p. value |
|
Zn (ppm) |
≤30 |
0.60+0.15 |
0.35+0.17 |
5.632 |
0.009 |
|
>30 |
0.47+0.17 |
0.74+0.11 |
7.253 |
0.003 |
|
Cu (ppm) |
≤30 |
0.96+0.26 |
0.95+0.25 |
0.966 |
0.989 |
|
>30 |
0.86+0.22 |
0.95+0.33 |
0.572 |
0.362 |
|
Se (ppm) |
≤30 |
0.22+0.09 |
0.29+0.05 |
1.365 |
0.075 |
|
>30 |
0.23+0.09 |
0.21+0.10 |
0.217 |
0.153 |
Significant P-value<0.05
Table (5): Comparison between serum level of zinc, copper, selenium
and age in alopecia areata groups
* There was a statistically significant decrease in Zn (group II) than
Zn (group I) when the duration of the disease is
≤
2 months (Table 6).
|
Duration/months |
Group I |
Group II |
t-test |
P- value |
|
Zn (ppm) |
≤2 |
0.53+0.17 |
0.34+0.17 |
4.256 |
0.017 |
|
>2 |
0.66+0.09 |
0.74+0.04 |
1.23 |
0.325 |
|
Cu (ppm) |
≤2 |
0.92+0.25 |
0.95+0.13 |
0.587 |
0.426 |
|
>2 |
0.96+0.28 |
0.94+0.32 |
0.965 |
0.258 |
|
Se (ppm) |
≤2 |
0.22+0.08 |
0.27+0.08 |
0.98 |
0.205 |
|
>2 |
0.23+0.14 |
0.23+0.10 |
0.993 |
0.969 |
Significant P-value < 0.05
Table (6): Comparison between serum level of zinc, copper, selenium
and duration of the disease in alopecia areata patients
* There were no significant relations between the studied trace elements
and the rest of clinical parameters.
Discussion
Alopecia areata is a disease of the anagen stage HFs with multifactorial
etiology and a strong component of autoimmune origin. AA is characterized
by either patchy hair loss or more generalized alopecia that results in
complete loss of scalp hair ( alopecia totalis) or body hair (alopecia universalis)
[14]. In addition to the disturbance of
the immune function, complex interactions between predisposing genetic and
environmental factors act as triggers for disease progression. Also, perifollicular
nerves and vasculature, viruses, trace element alterations, endocrine disorders
and thyroid dysfunction have been hypothesized. There are claims that imbalance
of trace elements may trigger the onset of AA[15].
In the present study we tried to evaluate and provide an insight about
the possible role of the trace elements, ( Zn, Cu and Se ) in the etiopathogenesis
of AA. The current study was carried out on 80 AA patients classified according
to number of lesions into group I (included 44 patients with single patch
of AA), group II ( included 36 patients with multiple patches of AA) and
20 healthy controls to evaluate the possible involvement of serum Zn, Cu
and Se in the pathomechanism of AA. Estimation of serum levels of the previous
trace elements was done by FAAS.
Alopecia areata in the present study, was presented in ages ranged from
(18-45) years with mean ± SD (29.5 ± 8.1) years and this was in agreement
with the previous studies [16] ,[17]
and disagreed with the study of another one which found that 70% of the
cases occurred between 10 and 25 year [18].
In the current study, AA affects both gender with male to female ratio 1:1
which is comparable to one study [19],
but other one has been observed that with relation to the severe forms,
63% occur in men and 36% in women[18].
A study conducted by Bhat et al 2009 [15]
showed an increased incidence of AA in men compared to women (17:8). In
the present study the duration of the disease, ranged from 1 to 6 months,
which is in agreement with the study conducted by Kato et al 1988[20],
in which the duration of the disease was found to be
≤
6 months in about the half of the 50 studied patients. In the present study,
positive family history of AA was seen in 5% of all the patients. One study
conducted on 206 AA patients revealed that the estimated lifetime risks
were 7.1% in siblings, 7.8% in parents and 5.7% in offspring [21]
,while some authors found positive family history in (8.4%) of 1032 AA patients
[22].
In the current study there was a statistically significant decrease in
the mean serum Zn level in both AA groups than controls and comparison between
the both AA groups revealed insignificant difference. These results were
in agreement with many previous studies, as they found statistically significant
low levels of Zn in AA patients compared to controls[15],
[20], [23],
[24]. Also, one study found a lower level
of Zn in blood and urine of children with AA than normal children(25). On
the other hand; Shin et al 2011 [26] found
significantly decreased serum level of Zn in patients, with different patterns
of hair loss including AA, when compared with controls while another study
did not find any difference in serum Zn concentrations of AA patients compared
to the normal population [12]. Some authors
stated that Zn deficiency causes the increase in the chemical messenger
TNF-α,
which causes the immune system to attack healthy tissues throughout the
body, including hair [27]. In the study
conducted by Park et al 2009 [28], 15 AA
patient were given Zn supplementation and after 12 weeks there was a statistically
significant increase in serum Zn level in the improved patients than those
with less improvement. It can be of quite significant clinical importance
since this trace element acts at molecular level and is active at very minute
concentrations. Zn deficiency induced by trace elements replacements with
heavy metals can cause the onset of alopecia besides other factors. Zinc
is an essential cofactor for multiple enzymes and it is involved with important
functional activities in the HF. Further, Zn is a potent inhibitor of HF
regression and it accelerates HF recovery.[29]
Hönscheid et al 2009 [30] concluded that
Zn homeostasis has a strong impact on T-lymphocytes. During Zn deprivation,
their development, polarization into effector cells and functional effectiveness
is impaired. Several molecular targets exist, including proteins involved
in the regulation of apoptosis, like Bcl-2/Bax and caspases. Additionally,
signal transduction involving protein kinase C and lymphocyte protein tyrosine
kinase is promoted by Zn and the inhibition of IL-1 receptor-associated
kinase leads to suppression of T-cell activity in response to Zn supplementation.
In the current study, there was a statistically significant decrease
in the mean serum Cu level in AA groups than controls and comparison between
both AA groups revealed insignificant difference, this was in agreement
with the study which found the serum levels of Cu were slightly lower in
AA patients than in healthy controls[23],
while another study found statistically insignificant difference in serum
Cu level between controls and patients with different patterns of hair loss
including AA [26]. Park et al 2007 [24]
found decreased serum level of Cu in cases of diffuse alopecia in women.
But this was in disagreement with the previous studies ,which they found
serum Cu level in patients was slightly increased than controls but it was
statistically insignificant [15,20]
On the other hand another study found that, the level of hair Cu and plasma
Cu in children with AA was significantly higher than normal healthy children
[31] . Amirinia et al 2011 [32]
found that low levels of Zn and Cu in the hair and serum may play a role
in the pathogenesis of AA and AGA. Another study interpreted the statistical
association of blood and serum levels of Zn, magnesium and Cu in patients
with many dermatological disorders including AA and after comparing with
healthy people; they did not find any change in serum levels of Zn and Cu,
but found a significantly higher level of magnesium. It has been suggested
that a possible reason for these different findings may be explained on
the basis of sample size, methodology and population variation [33].
As regards serum Se level, in this study there was a statistically significant
decrease in serum Se level in AA groups than control and comparison between
the both AA groups revealed non significant difference. This was in agreement
with one study which found that mean serum Se level was significantly lower
in AA patients, compared with control group, they did not observe any significant
association between the extent of AA and serum Se level [34].
Also previous study obtained low Se concentration in serum samples of a
few AA patients and suggested this fact may be as the result of low Se content
in their population foods. Se, an essential trace element, has immuno-modulating
and antiproliferative properties [12].
Se deficiency depresses the effectiveness of immune cells generally. Supplementation
with Se seems to boost cellular immunity by three mechanisms: first, it
up-regulates the expression of the T cell high-affinity IL-2 receptor and
provides a vehicle for enhanced T-cell responses; second, it prevents oxidative
stress-induced damage to immune cells; third, it alters platelet aggregation
by decreasing the ratio of thromboxane to leukotrine production. Measurement
of Se in plasma or serum is a frequently used method for assessment of Se
nutritional status.[34] Some authors stated
that when healthy and immuno-suppressed individuals were supplemented with
200 mcg/day of Se as sodium selenite for eight weeks showed an enhanced
immune cell response to foreign antigens compared with those taking a placebo[35].
A considerable amount of researche also indicates that Se plays a role in
regulating the expression of cell-signaling molecules called cytokines,
which orchestrate the immune response.[36]
one study found that, in Hashimoto's thyroiditis patients , selenomethionine
inhibited lymphocyte release of IL-2, INF-γ
and TNF-α,
which was accompanied by a reduction in plasma C- reactive protein levels,
this is suggesting probable role of Se in the etiopathogenesis of AA as
another autoimmune disease and also suggesting the expected benefit of Se
supplementation in treating AA[37]. Another
study reported that TNF-α
is well known to play a major role in the pathogenesis of AA, causing vacuolation
of matrix cells within the follicle bulb and a decrease in the size of the
matrix, as well as disorganization of follicular melanocytes and abnormal
differentiation and keratinization of the precortical cells and the internal
root sheath[14] .
In the present study, there was a statistically significant decrease
of Zn/Cu ratio in both AA groups when compared with controls, which is in
disagreement with the previous study which found insignificant difference
as regards serum Zn/Cu ratio in AA patients. Also, there was no statistically
significant difference in Zn/Se ratio and a statistically significant increase
in Cu/Se ratio in both AA groups than controls[20].
Another study which included 151 patients of various skin diseases including
AA, stated that Cu/Zn ratio showed significantly different values in those
patients, suggesting that the Cu/Zn ratio clearly reflects the severity
of the progress of the disease[38]. But
another found that, ratios of Cu/Zn were slightly higher in AA patients
than in healthy controls but their differences were not statistically significant[23].
In the current study, there was no significant difference between serum
trace elements and concerned clinical parameters in all AA groups as gender
(except for Zn and Cu, decreased in males of group II than group I, Se was
decreased in females of group II than group I), age (except for Zn in group
I and II), duration of the disease (except for Zn in group II when the duration
is
≤ 2 months, which disagreed with the one study which found more decreased
levels of Zn in those patient with prolonged duration and extensive lesions)
[15]. This may be due to relatively small
numbers of the studied cases. However, no available references in all literature
deal with all variables.
Conclusion
There was a significant decrease in the serum levels of the studied three
trace elements recorded in this study. There was no statistically significant
difference between AA and different clinical parameters ( age, gender, duration
of the disease and family history ) except in few of them so, in the view
of the small sample size, further investigations are needed. We suggest
that a serum Zn, Cu and Se assay should be included in the chemical assessment
of patients with AA and that trace elements ( Zn, Cu and Se ) supplementations
need to be given to AA patients who have low serum levels. The present study
suggests that the previous trace elements supplementation could become an
adjuvant therapy for the AA patients with low serum levels and for whom
the traditional therapeutic methods have been unsuccessful. The use of individualized
trace element supplementation is recommended than multiple ones. Study of
the trace elements level in AA patients before and after treatment to determine
its level with the prognosis of the AA. The current study advise for further
studies on the all trace elements in the serum and hair of AA patients.
References
1. MacDonald Hull SP, Wood ML, Hutchinson PE,etal. Guidlines
for the management of alopecia areata. Br J Dermatol 2003;692-699.
2. Rivetti EA. Alopecia areata: A revision and update. An
Bras Dermatol 2005;80:57-68.
3. Kuldeep CM, Himanshu S, Khare AK,etal. Randomized comparison
of topical betamethasone valerate foam, intralesional triamcinolone acetonide
and tacrolimus ointment in management of localized alopecia areata. Int
J Trichol 2011; 3: 20-24.
4. McDonagh AJ and Messenger AG. The pathogenesis of alopecia
areata. Dermatol Clin 1996;14:661-670.
5. McDonagh AJ and Messenger AG. The aetiology and pathogenesis
of alopecia areata. J Dermatol Sci 1994;7:S125-S135.
6. Hatfield DL and Gladyshev VN. How selenium has altered
our understanding of the genetic code. Mol Cell Biol 2002;22:3565-3576.
7. Huang X, Cuajungco MP, Atwood CS,etal. Alzheimer's disease,
β-amyloid
protein and zinc. J Nutr 2000; 130 :1488S-1492S.
8. Tapiero H and Tew KD. Trace elements in human physiology
and pathology: Zinc and metallothioneins. Biomed Pharmacotherapy 2003;57:399-411.
9. Tapieroa H, Townsend DM and Tew KD. Trace elements in
human physiology and pathology: Copper. Biomed Pharmacotherapy 2003;57:386-390.
10. Kotulanová
A and Komárek
J. Determination of copper in biological materials by atomic absorption
spectrometry. Acta Chim Slov 2002;49:437-445.
11. Filippou DK, Filippou G, Trigka A, etal. Malignant
proliferating trichilemmal tumour of the scalp. Report of a case and a short
review of the literature. Ann Ital Chir 2006;77:179-181.
12. Mussalo-Rauhama H, Lakomaa EL, Kianto U etal. Element
concentrations in serum, erythrocytes, hair and urine of alopecia patients.
Acta Derm Venereol 1986;66:103-109.
13. Welz B and Vale MGR. Analytical instrumentation handbook:
Atomic Absorption Spectrometry and Related Techniques 3rd edn. Cazes J and
Dekker M (eds.). New York 2005; chapter :7, p.75-125.
14. Gregoriou S, Papafragkaki D, Kontochristopoulos G,etal.
Cytokines and other mediators in alopecia areata. Mediat Inflamm 2010; 2010:
928030.
15. Bhat YJ, Manzoor S, Khan1 AR,etal. Trace element levels
in alopecia areata. Indian J Dermatol Venereol Leprol 2009;75:29-31.
16. Price. V. Alopecia areata: Clinical aspects. J Invest
Dermatol1991;96:685.
17. Camacho F. Alopecia areata: Clinical features. Dermatopathology.
In: Trichology: Diseases of the Pilosebaceous Follicle. Camacho F and Montagna
W (eds.). Madrid: Aula Medica Group 1997; p. 417-440.
18. Pimentel ERA. Alopecia areata. Aspectos imunologicos
e tratamento pelo DNCB. Universidade de Säo Paulo 1988.
19. Dawber RPR, de Beker D and Wojnarowska F. Disorders
of hair. In: Textbook of Dermatology. Champion RH, Burton JL, Burns DA and
Breathnach SM (eds.). Oxford : Blackwell Science 1998; p.2919-2927.
20. Kato AM, Baghagho SA and El-Maadawy IH. A study of
some trace elements profiles and cystine in patients with alopecia. Thesis
of Tanta University 1988; chapter 4, p. 83-99.
21. Blaumeiser B, van der Goot I, Fimmers R, etal. Familial
aggregation of alopecia areata. J Am Acad Dermatol 2006;54:627-632.
22. Yang S, Yang J, Liu JB, et al. The genetic epidemiology
of alopecia areata in China. Br J Dermatol 2004;151:16-23.
23. Lee SY, Nam KS, Seo YW,etal. Analysis of serum zinc
and copper levels in alopecia areata. Am Dermatol 1997;9:239-241.
24. Park JY, Lee JC, Jung HD, et al. Feasibility of clinical
and laboratory diagnostic approach to chronic female diffuse alopecia in
dermatologic outpatient clinic. Korean J Dermatol 2007;45:791-796.
25. Naginiene R, Kregzdyte R, Abdrakhmanovas A,etal. Assay
of trace elements, thyroid gland and blood indices in children with alopecia.
Trace Elem Electrol 2004;21:207-210.
26. Shin SJ, Yoo CS, Kill MS,etal. Analysis of serum zinc
and copper concentrations in hair loss patients. 63rd year of Korean Dermatology
Conference 2011; p.140.
27. Haase H and Rink L. The immune system and the impact
of zinc during aging. Immun Ageing 2009;6:9.
28. Park H, Kim CW, Kim SS,etal. The therapeutic effect
and the changed serum zinc level after zinc supplementation in patients
with alopecia areata who had a low serum zinc level. Ann Dermatol 2009;21:142-146.
29. Plonka PM, Handjiski B, Popik M,etal. Zinc as an ambivalent
but potent modulator of murine hair growth in vivo preliminary observations.
Exp Dermatol 2005;14:844-853.
30. Hönscheid A, Rink L and Haase H. T-Lymphocytes: A target
for stimulatory and inhibitory effects of zinc ions. Endocr Metab Immune
Disord Drug Targets 2009; 9:132-144.
31. Naginiene R, Abdrachmanovas O, Kregzdyte R,etal. Investigation
of heavy metal in people with alopecia. Trace Elem Electrol 2002;19:87-90.
32. Amirinia M, Sinafar S, Sinafar H,etal. Assessment of
zinc and copper contents in scalp hair and serum and superoxide dismutase,
glutathione peroxidase and malondialdehyde in serum of androgenetic alopecia
and alopecia patients. Med J Tabriz Univ Med Sci 2011;33.
33. Bruske K and Salfeld K. Zinc and its status in some
dermatological diseases: A statistical assessment. Z Hautkr 1987;62:125-131.
34. Feizy V, Mortazavi H, Barikbin B,etal. Serum selenium
level in Iranian patients with alopecia areata. JEADV 2008;22:1236-1278.
35. Kiremidjian-Schumacher L, Roy M, Glickman R,etal. Selenium
and immunocompetence in patients with head and neck cancer. Biol Trace Elem
Res 2000;73:97- 111.
36. Oguntibeju OO, Esterhuyse AJ and Truter EJ. Possible
benefits of micronutrient supplementation in the treatment and management
of HIV infection and AIDS. African J Pharm Pharmacol 2009;3:404-412.
37. Krysiak R and Okopien B. The Effect of levothyroxine
and selenomethionine on lymphocyte and monocyte cytokine release in women
with Hashimoto's thyroiditis. J Clin Endocrinol Metab 2011; 96:2206-2215.
38. Tasaki M, Hanada K and Hashimoto I. Analyses of serum
copper and zinc levels and copper/zinc ratios in skin diseases. J Dermatol
1993;20:21-24.© 2013 Egyptian Dermatology
Online Journal
|



