|
|
Abstract
Background: Skin tags are small skin colored to dark brown sessile
or pedunculated papillomas commonly occurring on the neck, axillae and the
eye lids, less often on the trunk and groin. Many theories were existing to
explain the occurrence of skin tags, some authors reported that skin
rubbing, skin aging and a familial predisposition are causes for skin tags,
while others described hormonal imbalances and hyper-insulinemia as
contributing factors. The association of skin tags with many diseases as
obesity, acromegaly, Crohn's disease and diabetes mellitus was proposed.
Objectives: Assessment of serum leptin, atherogenic lipids, HBAlC and
fasting blood glucose levels in patients with skin tags trying to find a
correlation between skin tags and the metabolic profile. Patients and
methods: 50 persons were included in this study, they were divided into
2 groups, the first group included 30 patients (each with 3 or more skin
tags) and the second group was age and sex matched 20 apparently healthy
volunteers with no skin tags as control. Patients and controls were
subjected to full history taking, dermatological examination, measurement of
body mass index and assessment of fasting glucose level, HbAlC, cholesterol,
triglycerides, HDL, LDL and serum leptin level which was measured by using
Solid Phase Sandwich ELISA. Results: Highly significant difference
was found of the fasting blood glucose, HbAIC, serum cholesterol, LDL and
VLDL between patients and controls while serum TG and HDL were higher in
patients than controls but did not reach the statistical significance. As
regard the serum leptin level we found a highly significant difference
between patients and control. Conclusion: Skin tags are associated
with increased serum leptin, hyperglycemia, increased HbAlC, dyslipidemic
lipid profile and obesity. Introduction
Skin tags are small skin colored to dark brown sessile or pedunculated
papillomas commonly occurring on the neck, frequently seen in the axillae
and the eye lids, less often on the trunk and the groin. Both sexes are
equally affected [1]. Histopathologically,
they are composed of loose collagen fibers and dilated capillaries [2].
Skin tags are generally ignored but they may represent a cosmetic concern.
Occasionally, they become irritated or necrotic and then they become
painful. In the last years, the finding of multiple skin tags has been
associated with abnormality of glucose metabolism especially type II
diabetes [3], and may be considered a
diagnostic clue to impaired carbohydrate metabolism. Atherogenic lipid
profile is thought to be strongly associated with atherosclerosis and
cardiovascular diseases and skin tags were found in association with
atherogenic lipid profile [4]. Also, skin
tags are cutaneous finding of metabolic disorder that comprise metabolic
syndrome (obesity, hypertension, dyslipidemia, insulin resistance and
hyperglycaemia)[5]. Leptin is a 16KDa
protein, is a product of obese gene (Ob), produced by adipocyte including
those of subcutaneous tissue. It is involved in the regulation of appetite
and energy expenditure[6]. Obesity and
aging can lead to increased leptin concentration due to dysfunctional leptin
signaling and not associated with reduced food intake and subsequent weight
loss, i.e. leptin resistance. This metabolic disturbance may have broad
physiological consequences affecting all body tissues including the skin.
Patients and Methods
Fifty persons were included in this study, with age ranged from 27 - 56
years old. They were divided into two groups, the first group included 30
patients (each with 3 or more skin tags) and the second group was age and
sex matched 20 apparently healthy volunteers with no skin tags as controls.
The patients were selected randomly from Out Patients Clinic of Dermatology,
Zagazig University Hospitals in the period between January and June 2011. We
excluded from our study: patients receiving any drug that could alter leptin
level as sympathomimetics, antipsychotics and retinoids. Patients receiving
any drug that could alter blood lipids (e.g. statins), pregnant females and
patients with thyroid function disorders were also excluded. Methods
All patients and controls were subjected to the following: Full
history taking: Including history of previous disease as diabetes
mellitus and thyroid disease, current pregnancy, any medication known to
alter leptin, glucose metabolism or blood lipids, smoking and special
habits. General examination: For detection of any systemic
diseases and measurement of blood pressure. Dermatological
examination: Including total body examination to detect any
associated skin disease, location and number of the skin lags.
Measurements of body mass index (BMI): BMI was calculated by the
following equation: BMI = 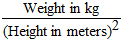 [7]
[7] Laboratory tests:
- Collection of samples: Venous blood (10ml) was taken after
twelve hours fasting, samples centrifuged at 4000rpm for 10 minutes, serum
samples were used for measuring fasting blood glucose level, HbAlc lipid
profile parameters including cholesterol, triglycerides (TG), HDL, LDL and
the rest of the samples were stored at - 80oC till the time of assay of
leptin when they thawed just before analysis. The following values were
considered normal as:
- Fasting blood glucose (FBG): 80 - 120mg/dL
- Cholesterol total < 200mg/dL
- Triglycerides < 150mg/dL
- HDL > 40mg/dL in
men.
- and > 50mg/dL in females.
- LDL < 100mg/dL
- HbAlc < 6.5% [8]
- Assessment of leptin levels: Measured by Solid Phase
Sandwich ELISA using a Commercial Kit (DGR leptin Sandwich ELISA E1A - 2395,
USA). Statistical analysis: They were done using
Statistical Program for Social Science (SPSS) version 12 as follow:
- Description of quantitative variables mean, SD and range.
- Description of
qualitative variables as number and percentage.
- Unpaired T-test to
compare quantitative variables between two groups.
- Analysis variance
(ANOVA) was used to compare qualitative data of three or more groups.
- Post HOC test was used in conjunction with ANOVA to determine which specific
group pair(s) is statistically different from each other.
- Chi-square
test was used to compare qualitative variables between groups.
- Fisher
exact test was used instead of Chi-square when one expected cell was less
than five.
- Spearman correlation test was used to rank different
variables versus each other positively or inversely.
(p) Values > 0.05 was
considered insignificant. (p) Values < 0.05 was considered significant.
(p) Values < 0.01 was considered highly significant. Results
The results of this study are illustrated in the following tables:
|
Variable |
ST patients |
Controls |
t |
(X2) |
P |
Sig. |
|
Number |
30 |
20 |
|
|
|
|
|
Gender: |
|
|
|
|
|
|
|
Female |
22 |
13 |
|
0.01 |
0.9 |
NS |
|
Male |
8 |
7 |
|
|
Age (years): |
|
|
|
|
|
|
|
Range |
30-56 |
27-36 |
1.71 |
|
0.09 |
NS |
|
Mean ± SD |
43.6 ± 7.96 |
39.1 ± 8.6 |
|
|
BMI: |
|
|
|
|
|
|
|
Range |
25.9 - 46 |
25 – 33.8 |
3.41 |
|
0.001 |
H Sig |
|
Mean ± SD |
32.8 ± 4.46 |
28.5 ± 2.97 |
|
Table (1): Characteristic of the study groups In table (1) there
were statistically non significant differences between patients and controls
as regards gender and age distribution, while a highly significant
difference in BMI was found between patients and controls.
|
Variable |
ST patients |
Controls |
t |
P |
Sig. |
|
Number |
30 |
20 |
|
|
|
|
F.B.G.: |
|
|
|
|
|
|
Range |
76 - 350 |
81 - 110 |
2.84 |
0.006 |
H Sig |
|
Mean ± SD |
155.7 ± 85 |
92 ± 9.5 |
|
HbAlc: |
|
|
|
|
|
|
Range |
5.2 – 11.4 |
4.8 – 6.5 |
3.39 |
0.0014 |
H. Sig. |
|
Mean ± SD |
10.94 ± 1.75 |
5.36 ± 0.5 |
Table (2): Fasting glucose and HbAlc In table (2) fasting blood
glucose and HbAlC were found to be highly significantly different between
patients and controls with high levels in patients.
|
Variable |
ST patients |
Controls |
t |
P |
Sig. |
|
Number |
30 |
20 |
|
|
|
|
Cholesterol: |
|
|
|
|
|
|
Range |
150 - 252 |
155 - 181 |
2.64 |
0.011 |
Sig |
|
Mean ± SD |
184.4 ± 27.5 |
164.8 ± 9.3 |
|
TG: |
|
|
|
|
|
|
Range |
80 – 189 |
93 – 181 |
0.2 |
0.83 |
NS |
|
Mean ± SD |
122.5 ± 35.7 |
120.2 ± 33 |
|
HDL: |
|
|
|
|
|
|
Range |
30 – 50 |
33.5 – 45.6 |
0.55 |
0.58 |
NS |
|
Mean ± SD |
39.7 ± 4.9 |
38.9 ± 45.6 |
|
LDL: |
|
|
|
|
|
|
Range |
95 – 175 |
86 – 157 |
2.2 |
0.03 |
Sig. |
|
Mean ± SD |
123.8 ± 24.4 |
106.3 ± 25.9 |
|
VLDL: |
|
|
|
|
|
|
Range |
17.6 – 40 |
17.6 – 29 |
3.75 |
0.001 |
H. Sig. |
|
Mean ± SD |
28.9 ± 6.6 |
21.8 ± 4.5 |
Table (3): Lipid profile in patients and controls In table (3)
the serum cholesterol, LDL and VLDL levels were significantly higher in
patients than controls, while serum TG and HDL levels were higher in
patients than controls but did not reach the statistical significant.
Regarding serum leptin, the highest levels were found in the patients with a
statistically highly significant difference between the groups as in table
(4).
|
Variable |
ST patients |
Controls |
t |
P |
Sig. |
|
Number |
30 |
20 |
|
|
|
|
Leptin: |
|
|
|
|
|
|
Range |
13.9 - 111 |
4 – 38.5 |
4.28 |
0.011 |
H. Sig |
|
Mean ± SD |
44.5 ± 23.1 |
16.5 ± 13.9 |
Table (4): Leptin level among study groups The comparison of
serum leptin levels between patients as regards the gender reveals a
significantly difference between females and males as in table (5).
|
Variable |
Females |
Males |
t |
P |
Sig. |
|
Number |
22 |
8 |
|
|
|
|
Leptin: |
|
|
|
|
|
|
Range |
18.5 - 111 |
13.9 – 42.2 |
2.41 |
0.02 |
Sig |
|
Mean ± SD |
50.2 ± 23.9 |
28.8 ± 10.8 |
Table (5): Relation between leptin and gender
|
Item |
r |
p |
Sig. |
|
Age |
0.31 |
> 0.05 |
NS |
|
BMI |
0.048 |
< 0.05 |
NS |
|
ST number |
-0.11 |
> 0.05 |
NS |
|
F.B.G. |
0.27 |
> 0.05 |
NS |
|
HbAlc |
0.17 |
> 0.05 |
NS |
|
Cholesterol |
0.01 |
> 0.05 |
NS |
|
TG |
0.01 |
> 0.05 |
NS |
|
HDL |
-0.29 |
> 0.05 |
NS |
|
LDL |
0.05 |
> 0.05 |
NS |
|
VLDL |
-0.04 |
> 0.05 |
NS |
Table (6): Correlation between leptin and other parameters Table
(6) shows a significant correlation between serum leptin levels and the mean
BMI with no significant correlations with other laboratory findings in
patients with skin tags. Discussion
Skin tags are very common benign dermal connective tissue neoplasm. They
usually occur as small, soft, pigmented or skin colored growth. Despite the
high incidence of skin tags, data about their etiopathogenesis are scarce in
the medical literature [9]. Because the
main etiology of skin tags is still obscure, Mc Coy 2008 [10]
proposed some risk factors that increase the chance to develop skin tags as
obesity pregnancy, polycystic ovarian syndrome, acromegaly, insulin
resistance, obesity, type II diabetes, Crohn's disease, skin shaving,
irritation and aging. Skin tags may be due to infection with human papilloma
virus. In the last years, skin tags was found to be associated with
abnormalities of glucose metabolism especially type II diabetes mellitus,
hyperinsulinemia and atherogenic lipid profile [4],
and also coexist in association with the components of metabolic syndrome [5].
Insulin is a hormone that promotes tissue growth and stimulates glucose
uptake in the tissues at an intensity that varies from one individual to
another, when insulin resistance is present the cells are less responsive to
the effect of this hormone and to compensate, the pancreas begins to produce
a greater amount of insulin (i.e. insulin resistance) [11].
Chronic hyperinsulnemia results in increased circulatory levels of insulin
growth factor-1 (IGF-1). Free IGF-1 is a potent mitogen for virtually all of
the body's tissues including the skin [12].
Leptin hormone may be related to the pathophysiology of insulin resistance
in both non-insulin dependent diabetes and obesity via inhibition of insulin
receptokinase activity and inhibition of phosphorylation of insulin receptor
substrate [13]. In our study, the two
groups (patients and controls) were evaluated according to the age, sex,
BMI, fasting blood glucose level, HbAlC, lipid profile and serum leptin
level. We found significant association between skin tags and increased BMI,
increased fasting blood glucose level, increased HbA1 C in agreement with
the results obtained by Tamega 2010 [14]
and in apposition with Gorpelioglue's results in 2009 [7],
who found no correlation between the presence of skin tags and these
findings. Comparing lipid profile in patients with skin tags and controls
in our study showed statistically significant differences between both
groups as regard serum cholesterol, LDL, VLDL levels with higher values in
patients than controls. These results were going on line with Gorpeligglu's
2009 [7] who found association between skin
tags and increased serum cholesterol and LDL. Assessment of serum leptin
level in the present work, revealed that the mean serum leptin level was
significantly increased in patients than controls. In contrast to
Gorpeligglu et al., 2009 [7] who found non
significant difference of serum leptin levels between patients and controls.
The increased serum leptin level in patients with skin tags than controls
can be explained by the proliferative effect of leptin in agreement with El-Safoury
et al., 2010 [15] who also found an
increased tissue leptin level in skin tags so, leptin may be involved in the
pathogenesis of skin tags through its proliferative effect. This is further
supported by Frank et al., 2000 [16] who
investigated the beneficial effect of leptin on keratinocytes in rodents.
Moreover, direct proliferative effects of leptin on mouse and human
keratinocytes have also been reported [12].
The proliferative effect of leptin may be due to its angiogenic effect and
induction of cellular proliferation as evidenced by El-Safoury et al., 2010
[15] who reported higher level of tissue
leptin in small skin tags compared to large ones, which may correspond to
increased proliferative potential of developing small skin tags compared to
more stable larger lesions. These results provided further support to the
proliferative effect of leptin. In this study, we found that females had a
significantly higher serum leptin level than males. This may be due to the
increase in the total fat mass in female bodies and also due to the effects
of sex hormones [17]. Serum leptin level in
skin tags patients is strongly correlated with BMI [18].
Our results going with these results, but in contrast Gorpelloglu et al.,
2009 [7] found a non significant difference
between groups as regard gender distribution. The relation between
increased BM1 and increased serum leptin level may be related to leptin
secretion by adipocytes and leptin receptors deficiency at the level of
hypothalamus and hyper leptinaemia. There was no correlation between serum
leptin level and lipid profile, glucose and HbA1C in our patients in
agreement with Pop et al., 2009 [19] who
found no correlation between HDL, cholesterol and leptin. Conclusion and
recommendations
The positive correlation between serum leptin levels, fasting blood
glucose, HbALC and the presence of skin tags in our study threw bright light
that patients with skin tags must be investigated for the presence of
metabolic abnormalities which are proved to be cardiovascular risk factors.
Increased serum leptin levels in patients of skin tags reflect the
proliferative and angiogenic effect of leptin which may have its role in the
stimulation of growth of different cell types or by stimulation of epidermal
growth factors production. Further studies including measuring serum leptin
level and epidermal growth factors in patients with skin tags both in skin
keratinocytes and skin tags is highly recommended for better understanding
of the role of leptin and its relation to many metabolic and endocrinal
abnormalities. We may propose that skin tags may be one of the important
skin markers of metabolic disorders and may attract physicians and
dermatologist for further investigation as it is proved to be not just a
cosmetic problem. References
1.
El-Safoury, O.; Fawzy, M.; El-Maadawa et al., (2009): Quantitation of mast
cells and collagen fibers in skin tags. Indian J. Dermatol., 54 (4): 319.
2. Canalizo-Almdisa, S.; Perez P. and Sanchez, A. (2007): Giant skin tags:
Report of two cases. Dermatol. Online J., 13: 30.
3.
Sudy, E.; Urbina, F.; Maliquco, M. et al., (2008): Screening of glucose
/insulin metabolic alteration in men with multiple skin tags on the neck.
Journal der Deutschen dermatologischen Gesellschoft., 6 (10): 852.
4. Dwivedi, S. and Jhamb, R. (2010): Cutaneous markers of coronary artery
disease. World J. Cardiol., 2 (9): 262.
5. Sari, R.;
Akman, A.; Alpsoy, E. et al., (2010): The metabolic profile in patient with
skin tags. Clin. Exp. Med., 10: 193.
6. Erdogan, S.;
Aktan, S.; Rota, S. et al., (2005): Skin tags and atherosclerotic risk
factors. J. Dermatol., 32 (5): 371.
7. Gorpelloglu,
G.; Erdal, E.; Ardicoglu, Y. et al., (2009): Serum leptin, atherogenic
lipids and glucose levels in patients with skin tags. Indian J. Dermatol.,
54 (1): 20.
8. Fauci, A.S.; Braunworld, E.; Kasper,
D. et al., (2009): Diabetes mellitus: Harrisons Manual of Medicine (17),
Chicago: McGraw-Hill; pp. 942.
9. Allegue, F.;
Fachal, C. and Perez, P. (2008): Friction induced skin tags. Dermatology
Online Journal. 14 (3): 18.
10. McCoy, K. (2008):
Acrochordons. EBSCO Dynamed webs.te. Available ati http/www. Ebscobhost.
Com/dynamed/what.php. Accessed December, 3, 2011.
11. Rasi, A.; Soltani-Arabshahi, R. and Shahbazi, N. (2007): Skin tags a
cutaneous marker for impaired carbohydrates metabolism. Int. J. Dermatol.,
46 (11): 1155.
12. Goren, I.; Pferlschifter, J. and
Frank, D. (2003): Determination of leptin signaling pathways in human and
murine keratinocytes. Biochem. Biophys. Res. Commun., 303: 1080.
13. Muller, G.; Fort, J.; Gerl, M. et al., (1997): Leptin impairs metabolic
actions of insulin in isolated rat adipocytes. J. Biol. Chem., 272 (16):
10585.
14. Tamega, A.; Aranha, A.; Guiotoku, M. et
al., (2010): Association between skin tags and insulin resistance. An. Bras.
Dermatol., 85 (1): 25.
15. El-Safoury, O.; Fawzi,
M.; Abdel Hay, R. et al., (2010): Increased tissue leptin hormone level and
mast cell count in skin tags: a possible role of adipoimmune in the growth
of benign skin growths. Indian J. Dermatol Venerol, Leprol., 76 (5): 538.
16. Frank, S.; Stallneyer, B.; Kanpfer, H. et al., (2000): Leptin enhances
wound re-epithelization and constitutes a direct function of leptin in skin
repair. J. Clin. Inves., 106: 501.
17. Flanagan,
D.E.; Vailej, C.; Pettey, G. et al., (2007): Gender differences in the
relationship between leptin, insulin resistance and the autonomic nervous
system. Regul. Rept., 140 (1 - 2): 37.
18. Cerman,
Aa.; Bozkurt, S.; Sava, S. et al., (2008): Serum leptin levels, skin leptin
and leptin receptor expression in psoriasis. British Journal of Dermatol.
159: 820.
19. Pop, D.; Zdrenghea, Distanca, L. et
al., (2009): Adiponectin and leptin levels correlate with body mass index
and lipid fractions but not with disturbances of glucose metabolism. Acta
Endocrinologica (Buc); V (3): 329.© 2013
Egyptian Dermatology Online Journal |

 [
[