|
|
Abstract
Background: The incidence of skin cancer and especially basal
cell carcinoma (BCC) has increased over the past decades. Current
management of basal cell carcinoma is surgical excision but in spite of
its high cure rate, the frequency of incomplete excision of basal cell
carcinoma varies widely (0.7-50%) among centers worldwide. The aim of
this case series was to assess the incidence of incomplete excision of
basal cell carcinoma as well as to evaluate the demographic
characteristics among 30 Syrian patients.
Methods: In this case series study, A total of 35 lesions (30
Syrian patients who had skin lesions suspected for BCC) which were
excised in the Aleppo University Hospital Clinic between March of 2008
to October of 2009 were studied. The following data: (Age, Sex, tumor
site and size, method of repair, histopathologic types and involvement
of surgical margins) were collected and analyzed by SPSS using
chi-square test and P value <0.05 was considered significant.
Results: There were 18 males and 12 females (40%) among Syrian
(BCC) patients. The mean age ± SD of the patients in the incomplete
excision group was (62.4 ± 3.0) years. The rate of incomplete excision
was 25.7% (9 lesions) and involvement of the deep margin was observed in
(55.5%) of these lesions. The most common sites for incomplete excision
were the ears, followed by the peri-ocular area, naso-labial folds and
the nose. Morphea type differentiation was associated with incomplete
excision (11.1%). Risk factors related to incomplete excision were
morphea type differentiation, repair by skin graft and lesions larger
than 20 mm in diameter. There was no statistically significant
differences in the distributions of the sex and age of the patients, and
the primary clinical diagnosis.
Conclusions: Basal cell carcinoma (BCC) appears to be on the
rise in our part of world. Careful clinical assessment, recognizing the
risk factors related to incomplete excision of BCCs and complete
excision with wide margins or Moh's micrographic surgery can avoid
recurrence and repeated surgeries.
Introduction
BCC is a slow-growing, locally invasive malignant epidermal skin
tumor predominantly affecting Caucasians [1].
It is the most common malignant tumor in humans, second in frequency
only to actinic keratosis if squamous cell carcinoma in situ is also
taken into consideration [2]. Metastasis
is extremely rare and morbidity results from local tissue invasion and
destruction particularly on the face, head and neck [3]
[4]. Clinical appearances and morphology
are diverse, and include nodular, cystic, superficial, morpheic
(sclerosing), keratotic and pigmented variants [5].
Prevalence of this tumor has increased over the past decades [1].
Current management of basal cell carcinoma is surgical excision but in
spite of its high cure rate, the frequency of incomplete excision of
basal cell carcinoma varies widely (0.7-50%) among centers worldwide [6]
[7]. Ten to forty % of incompletely
excised BCCs recur if left untreated, they present a therapeutic dilemma
[8]. The aim of this case series study
was to assess the incidence of this problem as well as to evaluate the
demographic characteristics among 30 Syrian patients. Knowing of these
factors helps the surgeons consider a wider excision margin or Moh's
micrographic surgery for high risk tumors for avoiding recurrence and
repeated surgeries.
Methods
In this case series, a total of 35 lesions (30 Syrian patients who
had skin lesions suspected for BCC) which were excised in the Aleppo
University Hospital (AUH) Clinic between March of 2008 to October of
2009 were studied. Excluded from the study were patients who underwent
incisional biopsies, shave biopsies or were previously treated by
irradiation or other modalities. Variables were age, sex, tumor site and
size and the method of repair. The operations were performed by
residents and consultant plastic surgeons. Before the operation, the
excision margins were defined through clinical judgment; after
considering the size, site and type of tumor. Most tumors were excised
with a margin of 4 mm and sent to the department of pathology at the
same center. The pathologic reports were reviewed to assess the adequacy
of the excisions, which was categorized into a dichotomous variable:
complete or incomplete excision. Incomplete excision was defined as a
pathologic report that indicated the presence of tumor cells at the
surgical margins of the lesion. Specimens with tumor cells only
approaching the surgical margins were not regarded as an incomplete
excision. Data were analyzed by SPSS (Statistical Package for Social
Sciences) using chi-square test and P value <0.05 was considered
significant.
Results
During the of 8 months, thirty patients (35 lesions) were studied.
There were 18 (60%) males and 12 (40%) females. The rate of incomplete
excision was 25.7% (9 lesions). In 55.5% ( 5 lesions) of the tumors,
deep margin and in (22.2%) of them, lateral margin was involved. Both
margins were involved in the rest. The mean age ± SD of the patients in
the incomplete excision group was (62.4 ± 3.0) years and in the complete
excision group was (60.8 ± 4.2) that was not statistically significant.
In men patients, the rate of incomplete excision was (27.8%) and in
women was (33.4%) that was not statistically significant (Table 1).
|
|
Complete excisions
(26 patients) |
Incomplete excisions
(9 patients) |
All excisions
(35 patients) |
|
Gender n (%) |
Men: 13 (72.2%)
Women: 8 (66.6%) |
Men: 5 (27.8%)
Women: 4 (33.4%) |
Men: 18 (60%)
Women: 12 (40%) |
|
mean age ± SD |
60.8 ± 4.2
|
62.4 ± 3.0
|
61.1± 5.1 |
Table 1. Demographic characteristics of patients with complete
vs incomplete excision of BCCs
Most tumors incompletely excised were located in the head and neck
region (22.5%). The most common sites were the ears, followed by the
peri-ocular area, naso-labial folds and the nose that was not
statistically significant. Lesions were divided into three groups
according to the parameter of size:
a) greater than 20 mm (44.4%),
b) lesions between 10 to 20 mm (33.3%) and
c) lesions smaller than 10 mm (22.3%) respectively that was
statistically significant.
Incomplete excisions were divided into three groups according to the
parameter of type of closure:
a) graft (27.2%),
b) flap (20%) and
c) direct closure (26.3%) respectively with a significant difference
(Fig 1).
Morphea type differentiation was associated with incomplete excision
but there was no association between incomplete excision and other
differentiation patterns (Table 2).
Differentiation
pattern |
Complete excisions
(n=26) |
Incomplete excisions
(n=9) |
|
Ulcerative |
7 (26.9%) |
3 (33.3%) |
|
Solid |
2 (7.6%) |
1 (11.1%) |
|
Morphea type |
1 (3.8%) |
1 (11.1%) * |
|
Baso- squamous |
2 (7.6%) |
1 (11.1%) |
|
Unspecified |
14 (53.8%) |
3 (33.3%) |
* p= 0.03
Table 2. Differentiation patterns of complete Vs incomplete
excisions of BCCs
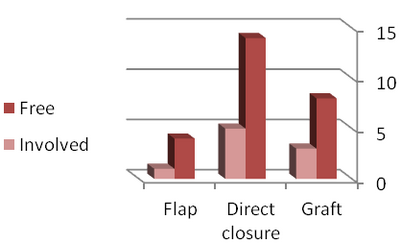 | Fig 1:
Frequency of incomplete excision by type of closure |
|
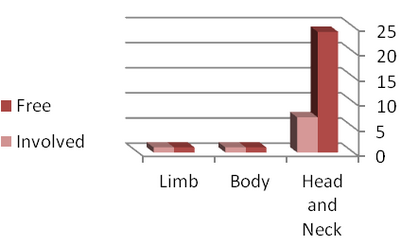 | Fig
2: Frequency of incomplete excision by site |
|
Discussion
In this study, we described the risk factors associated with
incomplete excision of BCCs performed by residents and consultant
plastic surgeons in the AUH clinic. The overall conventional surgical
excision of basal cell carcinoma has a high cure rate of (95-99%) [9].
Achieving this aim is possible when the excision is complete. The
frequency of incomplete excision of basal cell carcinoma varies widely
(0.7-50%) among centers worldwide [6] [7].
The incidence of incomplete excision was 25.7% in our study. The mean
age ± SD of the patients in the incomplete excision group was 62.4 ± 3.0
years similar to study data in Iran [10].
There was no statistically significant differences in the distributions
of the sex and age of the patients, consistent with most other series [10]
[11] [12]
[13] [14]
although they were significant in Kumar's study [15].
We have found that excision was incomplete at the deep margin in the
majority of the evaluated resections in contrast to studies with high
rates of incomplete excision at the lateral margin [7]
[15] [16]
[17]. We noted that 88.5% of all
lesions were located in the head and neck region that is similar to
series with reported incidence rates of 80-90% [13]
[17] [18].
The rate of incomplete excision was 22.5% for the head and neck region
which may be due to the inability of the surgeons to perform a wider
excision. This finding was similar to the study data in Iran [10].
The most common sites were the ears, followed by the peri-ocular area,
naso-labial folds and the nose that was not statistically significant
similar to Kumar's study [13]. Head and
neck region, especially mid-face is the site of highest incidence [15]
[16] [17]
[19]. This is the reflection of the
lack of enough skin and also cosmetic problems of resections. We have
found that incomplete excisions were significantly associated with the
presence of morphea type differentiation. This observation was
previously reported by many studies [13]
[14] [17]
[20]. It is possible that the more
aggressive BCCs, such as BCCs with baso- squamous, adenocystic or
morphea types of differentiation, are associated with increased
incomplete excision proportions because of the borders of this tumors
are not sharply demarcated. Lesions were greater than 20 mm in 31.4% of
cases and incomplete excision rate was 44.4% in this group which is
consistent with study data in Iran [10].
This might be due to the greater subclinical extension in larger lesions
[21] [22].
We have found that incomplete excision rate was higher in lesions
repaired by grafting (20%). This might be attributed to the fact that
larger, deeper and more complex lesions are repaired by grafting. Some
studies reported the highest rate of incomplete excision for grafting [10]
[14] [15].
In our study, the rate of incomplete excision was high for direct
closure repaired lesions which might show an inappropriate excision
margin. We tried to demonstrate the incidence of the incompletely
excised BCC lesions and to recognize the related variables to consider a
wider excision margin for high risk tumors because the recurrence rate
is 1% in completely excised lesions in contrast to 30% incomplete
excisions [23]. Some authors suggest
treating incompletely excised lesions in the immediate post operative
period to prevent extensive surgery [7]
[8] [20]
[23] [24].
Some other factors related to incomplete excision such as the level of
the expertise of the surgeons could not be assessed in this study and
need to be addressed in larger series.
References
1. Braun RP, Klumb F, Girard C et al. Three-dimensional
reconstruction of basal cell carcinomas. Dermatol Surg 2005; 31:562-6.
2. Tran H, Chen K, Shumak S. Epidemiology and aetiology
of basal cell carcinoma. Br J Dermatol. 2003;149 Suppl:50-2.
3. Lo JS, Snow SN, Reizner GT et al. Metastatic basal
cell carcinoma: report of twelve cases with a review of the literature.
J Am Acad Dermatol 1991; 24:715-19.
4. Ting PT, Kasper R, Arlette JP. Metastatic basal cell
carcinoma: report of two cases and literature review. J Cutan Med Surg
2005; 9:10-15.
5. Costantino D, Lowe L, Brown DL. Basosquamous
carcinoma - an under-recognized, high-risk cutaneous neoplasm: case
study and review of the literature. J Plast Reconstr Aesthet Surg 2006;
59:424-8.
6. Park AJ, Strick M, Watson JD. Basal cell carcinomas:
do they need to be followed up? JR Coll Surg Edinb 1994;36: 109-10.
7. Griffiths RW. Audit of histologically incompletely
excised basal cell carcinoma: recommendations for management by
reexcision. Br J Plast Surg 1999;52: 24-8.
8. Goldberg DP. Assessment and surgical treatment of
basal cell skin cancer. Clin Plast Surg 1997; 24: 673 - 686.
9. Dieu T, Macleod AM. Incomplete excision of basal cell
carcinomas: a retrospective audit. ANZ J Surg 2002; 72: 219-21.
10. Mirshams Shahshahani M, Razzaghi M, et al.
Incidence of incomplete excision in surgically treated basal cell
carcinomas and identification of the related risk factors. Iran J Derm,
Vol 14, No1, Spring 2011.
11. Goh Bk, Ang P, Wu YJ, Goh Cl. Characteristics of
basal cell carcinoma amongst Asians in Singapore and a comparison
between completely and incompletely excised tumors. Int J Dermatol 2006;
45, 561-4.
12. Bogdanov- Berezovsky A, Cohen AD, Glesinger R,
Cagnano E, Krieger Y, Rosenberg L. Risk factors for incomplete excision
of basal cell carcinomas. Acta Derm Venerol 2004; 84: 44-7.
13. Kumar P, Watson S, Brain AN, Davenport PJ,
McWilliam LJ, Banerjee SS, Bisset DL. Incomplete excision of basal cell
carcinoma: a prospective multicentre audit. Br J Plast Surg 2002; 55:
616- 22.
14. Su SY, Giorlando F, Ek EW, Dieu T. Incomplete
excision of basal cell carcinoma: A prospective trial. Plast Reconstr
Surg 2007; 120: 1240-8.
15. Kumar P, Orton CI, Mcwilliam LJ, Watson S.
Incidence of incomplete excision in surgically treated basal cell
carcinoma: a retrospective clinical audit. Br J Plast Surg 2000; 53:
563- 6.
16. Pascal RR, Hobby LW, Lattes R, Crikelair GF.
Prognosis of incompletely excised, versus, completely excised basal cell
carcinoma. Plast Reconstr Surg 1968; 41: 328-31.
17. Farhi D, Dupin N, Palangie' A, Carlotti A, Avril
MF. Incomplete excision of basal cell carcinoma: Rate and associated
factors among 362 consecutive cases. J Dermatol Surg 2007; 33: 1207-14.
18. Rippey JJ, Rippey E. Characteristics of
incompletely excised basal cell carcinomas of the skin. Med J Aust 1997;
166: 581-3.
19. Richmond JD, Davie RM. The Significance of
incomplete excision in patients with basal cell carcinoma. Br J Plast
Surg 1987; 40: 63-7.
20. Hauben DJ, Zirkin H, Mahler D, Sacks M. The
biologic behavior of basal cell carcinoma: Part I. Plast Reconstr Surg
1982; 69: 103 - 109.
21. Burg G, Hirsch RD, Konz B, Braun- Falco O.
Histographic surgery: accuracy of visual assessment of the margins of
basal cell epithelioma. J Dermatol Surg 1975; 1: 21-4.
22. Epstein E. How accurate is the visual assessment of
basal Carcinoma margins? Br J Dermatol 1973; 89: 37-43.
23. Robinson JK, Fisher SG. Recurrent basal cell
carcinoma after incomplete resection. Arch Dermatol 2000; 136: 1318-24.
24. Emmett AJ, Broadbent GG. Basal cell carcinoma in
Queensland. Aust NZ J Surg 1981; 51: 576- 90.© 2013 Egyptian Dermatology Online
Journal
|


