|
|
Abstract
Fixed drug eruptions (FDE) are common cutaneous adverse drug reaction
observed in clinical practice. Ornidazole, a 5-nitroimidazole antiamoebic
drug, is not a very well known cause of FDE. Few cases of ornidazole induced
FDE had been reported so far. However, another nitroimidazole antiamoebic
drug metronidazole is a common offender for causing FDE. We report a 37
year old female patient who developed severe pain and burning sensation
within the mouth about 3 to 4 hours after the intake of the first dose of
ornidazole. On examination it was found that the patient had developed FDE
in the oral cavity and lips. Next day she developed FDE in the palm of both
hands. Ornidazole was withdrawn and she was prescribed oral prednisolone
40 mg daily along with povidone iodine mouth wash. The lesions got resolved
in next 6 to 7 days. We found a probable association between this FDE and
ornidazole.
Introduction
Fixed drug eruption (FDE) is a type of allergic reaction to a medicine.
FDE are sharply marginated, round or oval itchy plaques of erythema and
edema becoming dusky violaceous or brown and sometimes vesicular or bullous.
It usually appears 30 minutes to 8 hours after administration of the offending
drug. The eruption may initially be morbilliform, scarlatiniform or erythema
multiforme like. However, urticarial, nodular or eczematous lesions are
uncommon.[1]
A fixed drug eruption characteristically occurs in the same site or sites
each time the offending drug is administered. However, with each exposure
the number of involved sites may gradually increase. Lesions are common
on the limbs than on the trunk. The hand, feet, genitalia and perianal areas
are the favoured sites. Perioral and periorbital lesions may occur also.
Genitalia and oral mucus membrane may be involved in association with skin
lesion, or alone.[1]
Usually, just one drug is involved, although independent lesions from
more than one drug had been reported. Cross sensitivity to related drugs
may occur. Different drugs have the adverse potential to cause FDE. Drugs
that are notorious for causing FDE are sulphonamides including cotrimoxazole,
oxyphenbutazone, metamezole, tetracyclines, piroxicam, aspirin, paracetamol,
ibruprofen etc. [1] Though FDE with metronidazole
is quite common, it is rarely noticed with ornidazole.
Although the exact pathophysiology of FDE is unknown, recent research
suggests a cell mediated process that initiates both the active and quiescent
lesions. The process may involve an antibody dependent cell mediated cytotoxic
response. CD8+ effector memory T cells play an important role in reactivation
of lesion with re-exposure to the offending drug. [2]
Ornidazole (C7H10C1N3O3) is an orally active 5-nitroimidazole
(1H-Imidazole-1-ethanol, α-(chloromethyl)-2-methyl-5-nitro-;
α-(Chloromethyl)-2-methyl-5-nitroimidazole-1-ethanol [16773-42-5]) used
for treatment of amoebiasis, giardiasis, trichomoniasis, anaerobic
infection and bacterial vaginosis [3]. Activity
of ornidazole is almost similar to metronidazole but ornidazole has longer
plasma half life compared to metronidazole [4].
Here we report a case of FDE which developed about 3 to 4 hours after intake
of the dose of ornidazole.
Case Report
A 37 year old female patient presented with complains of passage of loose
motions several times along with lower abdominal pain for two days. She
had moderate dehydration also. She was diagnosed as a case of amoebic dysentery.
She was prescribed tablet ornidazole 500 mg orally twice daily for five
days along with oral rehydration salt. About 3 to 4 hours after ingestion
of the first dose of ornidazole she experienced severe pain and burning
sensation within the mouth and in lips. She was unable to eat or drink anything
properly. On examination it was found that she had developed erythematous
and edematous well marginated ulcers within the oral cavity especially on
the distal part of the hard palate, angle of the mouth and on lips (fig
1). She was unable to open her mouth properly. She gave a past history of
one episode of ulceration in the oral mucosa after taking some tablets for
diarrhea but she was unable to specify that particular offending drug. Ornidazole
was stopped immediately and she was prescribed prednisolone tablets in a
dose of 40 mg daily orally along with povidone iodine mouth wash for 7 days.
Next day she developed sharply marginated erythematous blackish pigmented
plaques in the both palms (fig 2). However, improvement of the lesions
was observed within 24 hours after stopping the offending drug and starting
remedial medicines. The lesions within the mouth, lips and in palms of hand
get resolved in next 6 to 7 days.
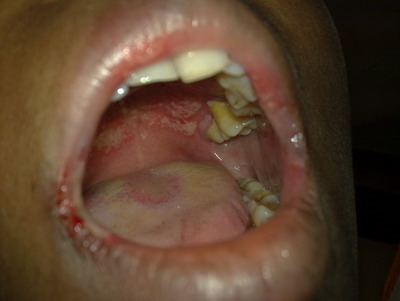 | Fig 1:
Erythematous ulceration with edematous swelling on mouth. |
|
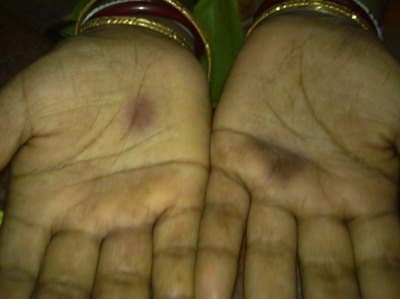 | Fig
2: Erythematous to hyperpigmented FDE in palms. |
|
Discussion
Cutaneous adverse drug reactions are one of the major obstacles in successful
pharmacotherapy. FDE are one of the most common cutaneous adverse reactions
we experience in our day to day life. FDE though not fatal, can cause enough
cosmetic embarrassment if present on the exposed part due to recurrence
of the reaction on previously affected site and residual hyperpigmentation
[5].
In the present case report the patient presented with FDE after intake
of ornidazole. Considering the cause of FDE, the probabilities were analyzed.
The patient was taking oral rehydration salt (ORS) along with ornidazole
and ORS was very unlikely to cause this FDE.
Causal relationship between the offending drug and the adverse drug reaction
mainly depends on the temporal association, dechallenge or rechallenge test
and inability to explain the adverse effect by another drug or disease [6].
The patient was able to specify the time of onset of FDE and that was about
3 to 4 hours after the intake of the first dose of ornidazole. The patient
showed positive response to dechallenge test as the lesions got resolved
after withdrawal of ornidazole. Earlier published work have already highlights
of ornidazole induced FDE worldwide [7],
[8], [9],
[10], [11]
but none of the scholars has been observed drug eruption in palm area in
hands. For the first time this study has reported drug eruption in both
hands which is unique for our report. FDE in this case could not be explained
by concomitant intake of ORS. The patient also stated that she had experienced
similar type of cutaneous reaction after intake of a drug but she was unable
to specify that particular offending drug.
The association between this FDE and ornidazole was evaluated using World
Health Organization (WHO) Uppsala Monitoring Centre (UMC) Causality Assessment
Criteria.[6] The WHO-UMC scale indicated
a probable association.
In conclusion, we can say that this type of case report may help us in
achieving desired therapeutic effect and to avoid unnecessary iatrogenic
health hazards.
References
1. Breathnach SM. Drug Reactions. In: Burns T, Breathnach
S, Cox N, Griffiths C, editors. Rook's Text Book of Dermatology. 7th ed.
Vol 4. Massachusetts: Blackwell Publishing. pp. 73.28-73.29, 2004.
2. Shiohara T. Fixed drug eruption: a prototypic disorder
mediated by effector memory T cells. Curr Allergy Asthma Resp. 9: 71-77,
2009.
3. Muller M. Action of clinically utilized 5-nitroimidazoles
on microorganisms. Scand J Infect Dis Suppl. 1981; 26: 31-41, 1981.
4. Rossignol JF, Maisonneuve H, Cho YW. Nitroimidazoles
in the treatment of trichomoniasis, giardiasis, and amebiasis. Int J Clin
Pharmacol Ther Toxicol. 1984;22:63-72, 1984.
5. Gupta R. Drugs causing fixed drug eruptions: Confirmed
by provocation tests. Indian J Dermatol Venereol Leprol, 69:120-121, 2003.
6. The use of WHO-UMC system for standardized case causality
assessment (monograph on the internet). Uppsala: The Uppsala Monitoring
Centre. Available from: http://www.who-umc.org/graphics/4409.pdf. [accessed
on July 29, 2010]
7. Gupta S, Jain VK, Aggarwal K, Mahendra A. Fixed drug
eruption caused by ornidazole. Contact Dermatitis. 53 (5):300-301, 2005.
8. Sanmukhani J, Shah V, Baxi S, Tripathi C. Fixed drug
eruption with ornidazole having cross sensitivity to secnidazole but not
to other nitroimidazole compounds. Brit J Clin Pharmacol. 69 (6):703-704,
2010
9. Gupta S, Mahendra A, Gupta S, Kaur S. Multiple fixed
drug eruption caused by ornidazole. Dermatitis. 21 (6): 330-331, 2010.
10. Marya CM, Sharma G, Parashar VP, Dahiya V. Mucosal
fixed drug eruption in a patient treated with ornidazole. J Dermatol Case
Rep 1: 21-24, 2012.
11. Baltaci D, Akyazi H, Kandis H, Saritas A, Mungan S,
Kara IH. Ornidazole-induced fixed drug eruption: A case report. Zdrav Vestn,
81: 337-340, 2012.© 2013 Egyptian Dermatology Online Journal
|


