|
|
Abstract
Dermoscopy is a surface skin microscopy technique that rapidly grew during
the past years enhancing the non invasive dermatological diagnostic techniques
effectively. Its main applications include classic dermoscopy, trichoscopy,
entomodermoscopy, inflammoscopy, capillaroscopy and dermoscopy for treatment
decision and monitoring.
Introduction
Dermoscopy is a non-invasive technique that allows a rapid and magnified
in vivo observation of the skin with the visualization of morphologic features
invisible to the naked eye [1]
Benefits[2]
- Helps to differentiate melanocytic from non-melanocytic skin lesions.
- Helps to differentiate benign from malignant skin lesions.
- Increases the diagnosis of early melanoma.
- Increases the diagnosis of melanoma incognito.
- Helps to avoid unnecessary surgery.
- Helps to plan surgery.
- Helps one to work better with their pathologist (asymmetrical high
risk criteria, collision tumors, dermoscopic-pathologic correlation).
- Patient reassurance.
- Allows for follow up of patients with multiple nevi digitally to
find changes over time.
Parts[3]
- Magnifier (10x).
- Non-polarized light source.
- Transparent plate.
- Oil or fluid is placed on the lesion as fluid eliminates reflection
of light from the surface of the skin allowing visualization of color
and structure down to the papillary dermis (Fig 1) [4].
- Recently, new systems utilizing polarized light may achieve similar
results without the need for liquids (Fig 1) [4].
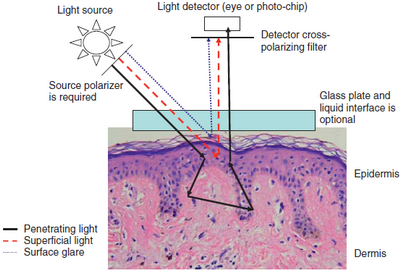
| Fig 1: Optics of polarized & non-polarized light in dermoscopy
[4] |
|
Applications:
(I) Classic dermoscopy
It aids in the diagnosis of pigmented and non-pigmented skin tumors including
melanocytic, non-melanocytic, benign, and malignant skin tumors. There are
two major approaches; the Heuristic approach and the Analytical approach.
The Heuristic approach is called "The Pattern Analysis" and utilizes a 2
step algorithm (Fig 2). In the first step of decision making, the
physician decides whether a lesion is of melanocytic or non-melanocytic
origin through the following seven steps [5]
 | Fig
2: The Pattern Analysis (2 Step Algorithm) [4] |
|
- Level 1: Criteria of Melanocytic Lesions
The lesion is considered of melanocytic origin if any structure of the
following is present; pigment network (Fig 3.a), branched streaks
(Fig 3.b), streaks, negative network (Fig 3.c), aggregated
globules (Fig 3.d), homogenous blue pigmentation (Fig 3.e),
pseudo-network (face) (Fig 3.f), or parallel pattern (palms, soles,
and mucosa) (Fig 4).
Diagnosing the lesion as pigmented in origin allows the physician to
proceed to step two to evaluate whether it is benign, suspicious, or malignant.
Dermatofibroma can be
also diagnosed at this level, characterized by multiple patterns, the
most common of them is a central white structureless area surrounded by
fine peripheral pigment network.
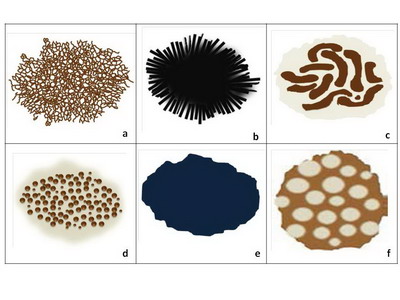 | Fig 3: Criteria of melanocytic lesions [6] |
|
 | Fig 4: Benign and malignant patterns of volar areas (ALMM= acral
lentigenous malignant melanoma) [7] |
|
- Level 2: Criteria for basal cell carcinoma
Search for the presence of specific morphologic criteria for basal cell
carcinoma which include arborizing blood vessels, leaf-like areas, large blue-gray
ovoid nests, multiple blue-gray non-aggregated globules, spoke-wheel-like
structures, shiny white areas, or ulceration (Fig 5). [8]
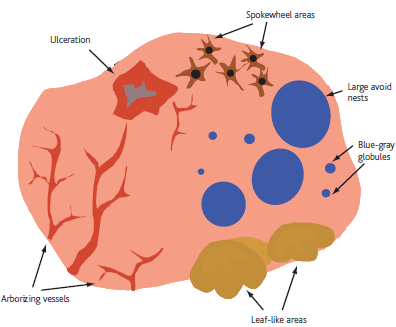 | Fig 5: Diagram showing BCC dermoscopic features [6] |
|
- Level 3: Criteria for seborrheic keratoses
Look for multiple milia-like cysts, comedo-like openings, crypts, moth-eaten
borders, network-like structures, "fissures and ridges" that sometimes give
a brain-like or cerebriform appearance to the lesion, fat finger like structures),
or light brown fingerprint-like structures (Fig 6).[9]
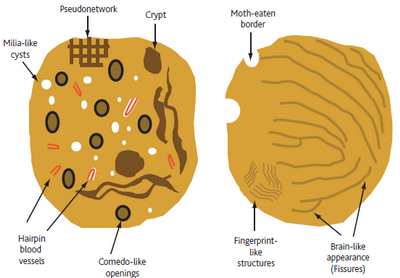 | Fig 6: Diagram showing dermoscopic features of seborrheic keratosis
[6] |
|
- Level 4: Criteria for vascular lesions
The presence of red, maroon, or red-blue to black lacunae (also known
as lagoon-like structures), indicates that the lesion is a haemangioma or
angiokeratoma (Fig 7).
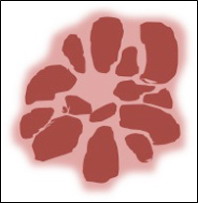 | Fig
7: Diagram showing dermoscopic features of hemangioma [10] |
|
- Level 5: Specific blood vessels in non-melanocytic lesions
If none of the morphologic criteria described in the previous levels
can be identified, then the lesion is considered amelanotic or hypomelanotic.
In such lesions, the physician may be able to find vascular structures that
can assist in the diagnosis. It is important to observe both the morphology
and distribution of the vessels. Hairpin vessels surrounded by a whitish
halo is a characteristic of keratinizing tumors, such as keratoacanthoma
and seborrheic keratosis (Fig 8a). The presence of glomerular vessels,
usually aggregated focally at the periphery of the lesion identifies the
lesion as a squamous cell carcinoma or bowen's disease (Fig 8b).
The presence of blood vessels arranged like "string of pearls" is a hallmark
of clear cell acanthoma (Fig 8c) while the presence of crown vessels
identifies the lesion as a sebaceous hyperplasia or molluscum contagiosum
(Fig 8d) [5]
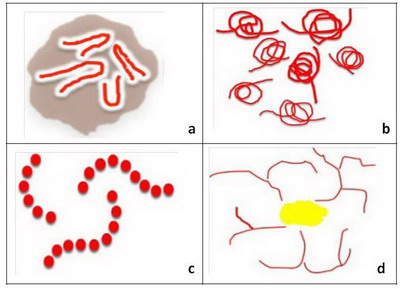 | Fig 8: Diagram showing different vascular patterns seen in non-melanocytic
lesions [2] |
|
- Level 6: Specific blood vessels in melanocytic lesions
The presence of predominantly comma shaped blood vessels is a hallmark
of intradermal nevi. The blood vessel morphology encountered in melanoma
includes dotted (Fig 9a), linear irregular (Fig 9b), atypical
hairpin (serpentine) vessel in a pink background, and cork screw (Fig
9c), tortuous vessels or milky red areas (Fig 9d) . If more than
one type of vessel morphology is seen within the same lesion, the vascular
pattern is termed "polymorphous" (Fig 9e), which is the most common
pattern associated with melanoma. Lesions that do not display any of the
structures mentioned in steps 1-6 are considered "structureless" and for
such lesions one needs to proceed to level 7 (Fig 10) [5]
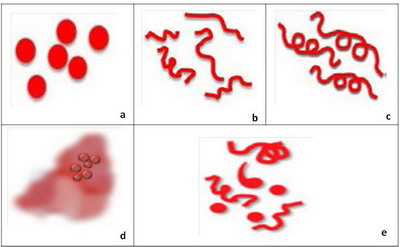 | Fig 9: Diagram showing different vascular patterns seen in melanocytic
lesions [2] |
|
- Level 7: "Structureless" lesions
This category includes all lesions that fail to reveal any specific diagnostic
structures to help classify them as melanocytic or as one of the non-melanocytic
lesions. For example, the presence of fine dots, peppering, blue white veil,
crystalline structure, and blotches may be present in these lesions. Although
these structures cannot be used to differentiate melanocytic from non-melanocytic
lesions, they can be clues that aid in correctly identifying melanomas and
some basal cell carcinomas. These lesions either should be biopsied or should
be subjected to short-term mole monitoring in an attempt to ascertain their
biologic behavior [5]
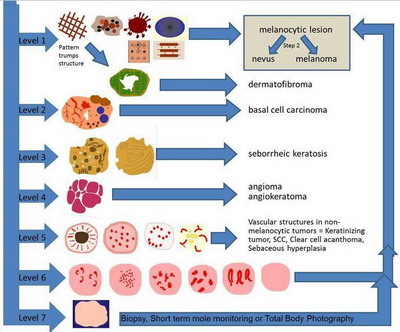 | Fig 10: Summary of Pattern analysis (1st step) [6] |
|
The second step of decision making is deciding whether the melanocytic
lesion is benign, suspect or melanoma. To accomplish this, many different
approaches have been proposed.
Revised 7- point checklist (Fig 11) [11]
The seven-point checklist is based on the detection of seven dermoscopic
features commonly associated with melanoma
- Atypical pigment network,
- Blue whitish veil,
- Atypical vascular pattern,
- Irregular streaks,
- Irregular dots or globules,
- Irregular blotches,
- Regression structures.
A score is calculated by summing points giving one point for each criterion.
If the lesion scores 1 or more, then it should be examined carefully as
it's suspicious for melanoma.
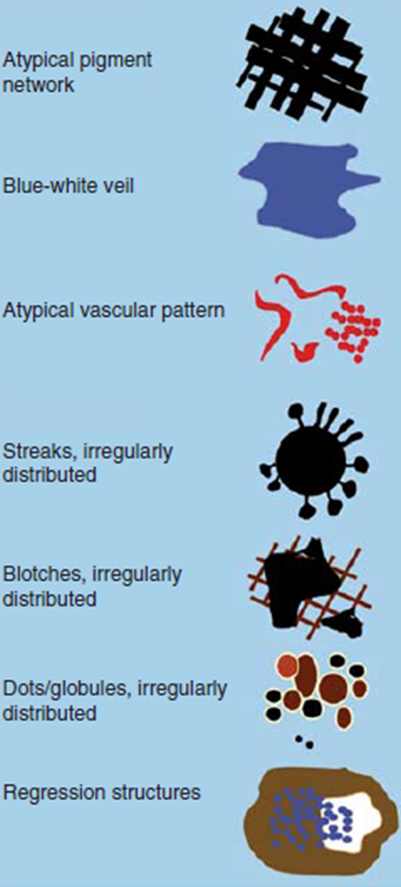 | Fig 11: The revised seven point checklist [12] |
|
Three-Point Checklist [13]
- Asymmetry: is defined as asymmetry in the distribution of dermoscopic
colors and/or structures in one or two perpendicular axes.
- Atypical network: is defined as pigmented network lines that are
focally thickened and distributed in an irregular or disorganized manner.
- Blue-white structures: includes any blue and/or white color visible
within the lesion.
The presence of more than one of the above three criteria is suggestive
of
a malignancy (basal cell carcinoma or melanoma).
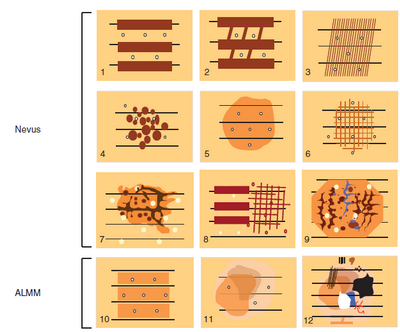
Pattern analysis [14]
Benign nevus patterns include: (Fig 12)
- Diffuse reticular,
- Patchy reticular,
- Peripheral reticular with central hypopigmentation,
- Peripheral reticular with central hyperpigmentation,
- Peripheral reticular with central globules,
- Globular,
- Peripheral globules,
- Starburst,
- Homogenous,
- Two component,
- Symmetrical multicomponent.
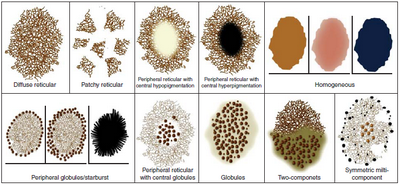 | Fig 12: Diagram showing Dermoscopy benign nevus patterns [4] |
|
Melanoma specific features include: (Fig 13)
- Atypical pigment network,
- Streaks (Radial streaming and pseudopods),
- Negative pigment network,
- Crystalline structures,
- Atypical dots, and globules,
- Off centered blotches,
- Regression structures,
- Blue white veil on raised areas,
- Atypical vascular structures,
- Peripheral brown structureless areas.
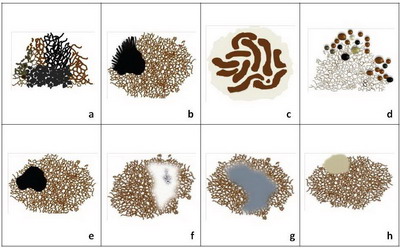 | Fig 13 :Diagram showing melanoma specific features [4] |
|
Revised pattern analysis [15] (Analytical
approach)
Basic Elements (Fig 14)
All patterns observed in dermoscopy are composed of
five simple geometric elements. These basic elements are:
- Lines
- Dots
- Clods
- Circles
- Pseudopods
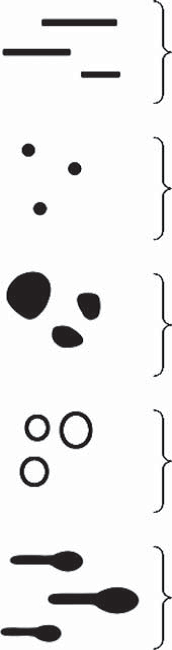 | Fig 14: The basic 5 dermoscopic structures [15] |
|
Patterns Formed by Basic Elements (Fig 15,16)
A pattern is built up of multiple repetitions of the same single basic
element. There is also a pattern "structureless," an area characterized
by the absence of any of the basic elements, or at least with no basic element
dominating. Lines can be arranged to form five different patterns: reticular,
branched, parallel, radial, and curved.
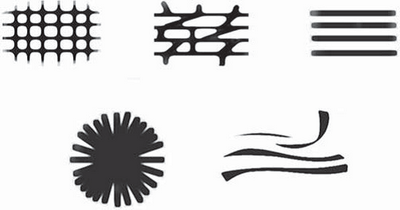 | Fig 15: The dermoscopic lines patterns [15] |
|
 | Fig 16
: Dermoscopic basic structures patterns [15] |
|
Colors
Colors seen in dermoscopy are created by various combinations of keratin,
melanin, blood (including serum in crusts), collagen, and foreign material.
The color of melanin varies greatly depending on localization in the epidermis
or dermis (Fig 17).
 | Fig 17: Different colors seen in dermoscopy [15] |
|
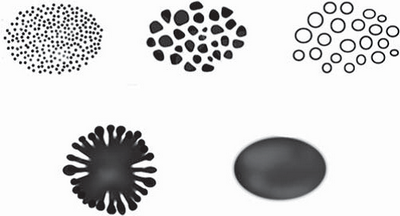
Vessels
Morphology: (Fig 18)
- Dots (A)
- Clods (B)
- Lines
- Straight (C)
- Looped (D)
- Curved (E)
- Serpentine (F)
- Helical (G)
- Coiled (H)
 | Fig 18: The basic dermoscopic vascular structures[15] |
|
Distribution (Fig 19)
- Random (A)
- Clustered (B)
- Serpiginous (C)
- Linear (D)
- Centered (E)
- Radial (F)
- Reticular (G)
- Branched (H)
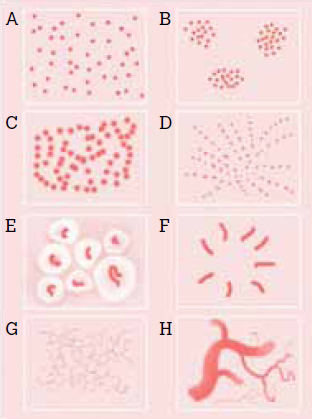 | Fig
19: The basic dermoscopic vascular patterns[15]
|
|
The analytical approach is based on the Chaos & Clues method, any pigmented
skin lesion is then classified into either chaotic or not based on its color,
pattern but not on outline or shape. If the lesion is not chaotic, then
no further intervention is needed, but if chaotic then, you should search
for a clue for the diagnosis of melanoma which are:
- Grey or blue structures
- Eccentric structureless areas
- Thick reticular lines
- Black peripheral dots or clods
- Radial lines or pseudopods (segmental)
- White lines
- Parallel ridge lines
- Polymorphous vessels
- Polygons
The only exceptions for this rule is the growing nodules in adults as
well as facial lesions with any grey colour and acral volar lesions with
parallel ridge pattern.
ABCD rule (Table 1) [16]
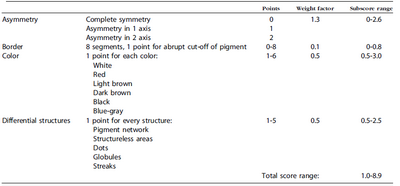 | Table 1: The ABCD rule of dermoscopy |
|
Menzies method [17]
To diagnose melanoma, both of the following negative features must not
be found: a single color or point and axial symmetry of pigmentation. Additionally,
at least one positive feature of the following must be found; blue-white
veil, multiple brown dots, pseudopods, radial streaming, scar-like depigmentation,
peripheral black dots or globules, multiple colors (5 or 6), multiple blue/gray
dots or broadened network.
(II) Trichoscopy: [18]
It is that branch of dermoscopy dealing with hair and scalp disorders.
It is rapidly gaining acceptance in the diagnosis and follow up treatment
of cicatricial and non cicatricial alopecias. It can be used also in diagnosing
different hair shaft disorders as well as infections and infestations.
Applications:
Non- cicatricial alopecia: (Fig 20, 21)
Alopecia areata
The hallmark trichoscopic features of alopecia areata are regularly distributed
yellow dots, micro-exclamation mark hairs, tapered hairs, black dots, broken
hairs, and upright and regularly coiled (circle, pigtail) re-growing hairs.
Trichorrhexis nodosa may be observed, especially in active, early alopecia
areata. It must be emphasized that micro-exclamation mark hairs are not
pathognomonic for alopecia areata. Trichoscopy of alopecia areata may differ
depending on the disease activity, severity and duration.
|
Active hair loss |
Long standing inactive disease |
Hair regrowth |
|
Black dots |
Yellow dots |
Upright re-growing hair |
|
Micro-exclamation marks |
Vellus hair |
Vellus hair |
|
Broken hair |
Follicular openings may not be visible |
Pigtail hair |
|
Monilethrix like |
|
|
|
Trichorrhexis nodosa |
|
|
Table 2: Trichoscopic features of alopecia areata
Male and female AGA share similar trichoscopic features, including hair
shaft thickness heterogeneity, thin hairs, yellow dots, perifollicular discoloration
(the peripilar sign), an increased proportion of vellus hairs, and a large
number of follicular units with only one emerging hair shaft. Thin wavy
hair and honeycomb hyperpigmentation often coexist as additional, unspecific
features. These features are more pronounced in the frontal compared to
the occipital region.
Telogen effluvium
Trichoscopy has limited value in diagnosing telogen effluvium. Frequent,
but not specific, findings include the presence of empty hair follicles,
a predominance of follicular units with only one hair, perifollicular discoloration
(the peri-pilar sign), and upright re-growing hairs with lack of features
of any other disease. There is no significant difference between the findings
in the frontal area and those of the occipital area, which differentiates
telogen effluvium from androgenetic alopecia.
Trichotillomania
Trichoscopy of trichotillomania is characterized by a chaotic pattern
of diverse features related to hair fracturing. The most characteristic
features are hairs broken at different lengths, short hairs with trichoptilosis
(split ends), irregular coiled hairs, amorphous hair residues, and black
dots.
Tinea capitis
Dermoscopy of tinea capitis shows two typical features; comma hairs (curved
fractured hair shafts) and corkscrew hairs. Broken and dystrophic hairs
also are seen.
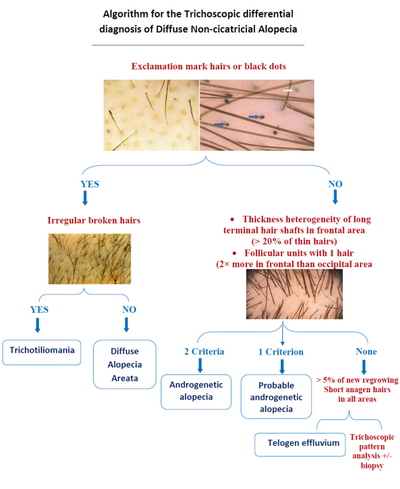 | Fig 20: Algorithmic approach for the trichoscopic diagnosis of
diffuse non-cicatricial scalp alopecia [18] |
|
 | Fig 21: Algorithmic approach for the trichoscopic diagnosis of
focal non-cicatricial scalp alopecia [18] |
|
Cicatricial alopecias (Fig 22)
Lichen planopilaris
Trichoscopic features of active lesions include; perifollicular scaling
(scales entangling hair shafts up to 2-3 mm above the scalp surface in a
tubular manner), hair casts, elongated linear blood vessels and violaceous
areas. Inactive lesions show irregular, large white dots (fibrotic white
dots), white areas, milky red areas (strawberry ice cream color) and tufted
hairs.
Frontal fibrosing alopecia
Scalp lesions show lack of follicular openings, homogenous ivory-colored
background, minor perifollicular scaling, perifollicular erythema, follicular
openings with only one hair at the hair-bearing margin, perifollicular brown
or brown-violet areas (in dark-skinned patients). While eyebrows lesions
show multiple regularly distributed red dots in early phase of disease and
multiple regularly distributed red or gray to gray-brown dots in advanced
cases.
Discoid lupus erythematosus
Active (early) lesions show thick arborizing vessels, large yellow dots
corresponding to the follicular keratotic plugs, fine interfollicular scaling,
scattered brown discoloration, red dots, blue-gray dots (on dark or sun-exposed
skin). On the other hand, inactive (end-stage) lesions show loss of follicular
openings, pink areas, white areas, arborizing vessels and yellow dots containing
thin spider vessels (in prefibrotic lesions).
Folliculitis decalvans
Active disease show tufts of five or more hairs in one follicular unit,
yellow follicular pustules, yellowish tubular scaling with collar formation,
yellow discharge, starburst sign corresponding to folds of epidermal hyperplasia
with elongated looped or coiled vessels arranged in concentric perifollicular
pattern. Late stage (inactive disease), milky red areas lacking follicular
openings or white areas lacking follicular openings are usually seen.
Dissecting cellulitis
Trichoscopic features of active dissecting cellulitis include; yellow
structureless areas, yellow 3D (soap bubble) dots with or without hair shafts,
black dots, pinpoint-like vessels with a whitish halo, cutaneous clefts
with emerging hairs and hair tufts.
Pseudopelade of Brocq
Trichoscopic features of pseudopelade of Brocq include; no follicular
openings, smooth white areas, dystrophic hairs at the periphery of the lesion
with no features indicative of other types of cicatricial alopecia.
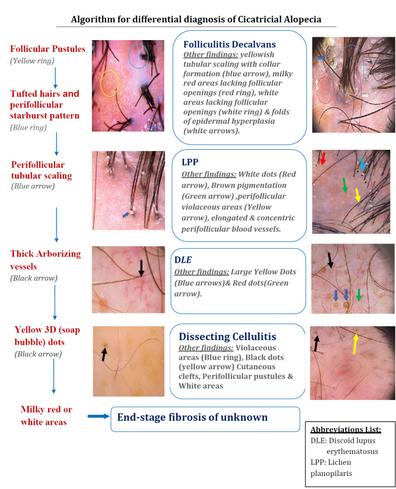 | Fig 22: Algorithmic approach for the trichoscopic diagnosis of
focal cicatricial scalp alopecia [18] |
|
Hair shaft abnormalities
They can also be seen as in cases of fractured hair in the form of: trichoptilosis,
trichoschisis/trichoclasis and Irregular fractures caused by mechanical
force or trichorrhexis invaginata, narrowing as in cases of monilethrix,
monilethrix-like congenital hypotrichosis, monilethrix-like hairs (Pohl-Pinkus
constriction), pseudomonilethrix, tapered hairs and exclamation mark hairs,
node-like appearance as in cases of trichonodosis, trichorrhexis nodosa,
Bamboo hairs (trichorrhexis invaginata) and hair casts, curls and twists
as in pigtail hairs (circular or oval), coiled hairs, comma hairs, corkscrew
hairs, z-hairs (zigzag hairs), pili torti or woolly hairs, bands as in cases
of interrupted medulla, pili annulati and interrupted (Morse code-like)
hairs and short hairs as in cases of upright re-growing hairs, vellus hairs,
tulip hairs, block hairs, i-hairs, broom hairs and flame hairs.
(III) Entomodermoscopy:
It is the one diagnosing skin infections and infestations
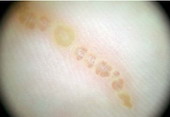
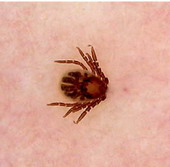
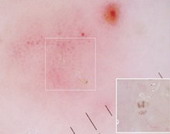
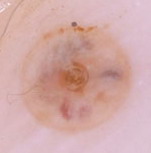
Fig23: Different types of skin infections and infestations detected
by dermoscopy F,I J,K [19], A,B,C,D,G,L,M
[29]
(IV) Inflammoscopy:
This is the one involved with inflammatory skin diseases.
|
Diagnosis |
Dermoscopic features |
|
Lichen planus |
Radially arranged, horizontally
oriented red lines or red dots surrounding reticular whitish
striae (Wickham’s striae)
[30]. |
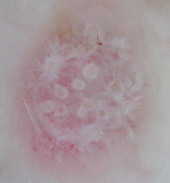
A |
|
Psoriasis |
Regularly distributed dotted
vessels and scales
[31]. |
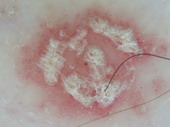
B |
|
Pityriasis rosea |
Central mixed vascular pattern and peripheral collaret of
scales [32]. |
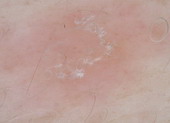
C |
|
Eczema |
Dotted vessels in a patchy arrangement and yellow scales.[33] |
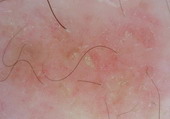
D |
Fig 24: Different types of inflammatory skin diseases detected
by dermoscopic examination A,B,C,D [33]
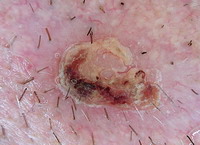
(V) Capillaroscopy:
It evaluates the nail fold capillaries for the screening of autoimmune
diseases.
|
Diagnosis |
Definition/diagnostic significance |
|
Scelroderma Dermatomyositis
(SD) pattern[34] |
Two or more of the following
patterns in at least two nail folds:
- Enlargement of the capillary loops
- Loss of capillaries
- Disorganization of the distribution of the
capillaries
- Budding capillaries
- Twisted capillaries and capillary hemorrhages
(extravasates)
|
|
Dermatomyositis[35] |
SD pattern |
|
Lupus erythematosus[35] |
SD pattern/mixed vascular
pattern including
giant capillaries or loss of capillaries |
|
Mixed Connective Tissue Disease
(MCTD)
[35] |
SD pattern |
Raynaud phenomenon
without evidence of
underlying CTD[34]
|
SD pattern/twisted capillaries
and extravasation
of erythrocytes |
|
Scleroderma[36] |
SD pattern |
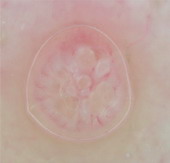
Fig 25 : Dermoscopic examination of nail fold capillaries in scleroderma
patient showing enlarged capillary loops with hemorrhage [33]
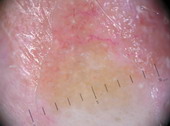
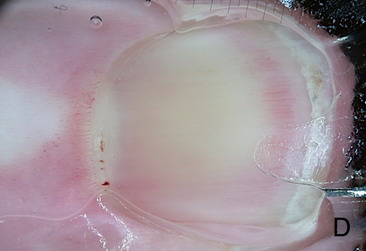
(VI) Dermoscopy for treatment decision and monitoring: [33]
It is used for taking decisions preoperatively regarding lesions margins
and extension, and in follow up especially after non-invasive treatment
modalities.
|
|
|
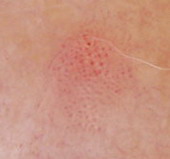
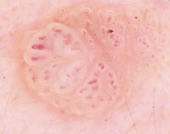
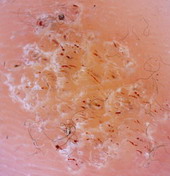
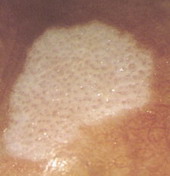
| Fig 26. Dermoscopy of BCC before and after topical treatment [33] |
|
References
1. Lacarrubba F, D'Amico V, Nasca M R, Dinotta F, Micali
G. Use of dermatoscopy and videodermatoscopy in therapeutic follow-up: a
review. International journal of dermatology, 2010, 49(8): 866-873.
2. Marghoob A, Usatine R P, Jaimes N. Dermoscopy for the
family physician. Am Fam Physician, 2013, 88: 441-450.
3. Wang S Q, Marghoob A A, Scope A. Principles of dermoscopy
and dermoscopic equipment. An Atlas of Dermoscopy, 2012. Informa Healthcare,
London.
4. Marghoob A. Pattern analysis. Atlas of Dermoscopy, 2012.
Informa Healthcare, London.
5. Argenziano G, Soyer H P, Chimenti S, Talamini R, Corona
R, Sera F, Binder M. Dermoscopy of pigmented skin lesions: results of a
consensus meeting via the Internet. Journal of the American Academy of Dermatology,
2003, 48(5): 679-693.
6. Marghoob A, Braun R P. Two-step algorithm: Differentiating
melanocytic from nonmelanocytic lesions. Atlas of Dermoscopy, 2012. Informa
Healthcare, London.
7. Malvehy J, Puig S. Special locations. Atlas of dermoscopy,
2012. Informa Healthcare, London.
8. Menzies S W, Westerhoff K, Rabinovitz H, Kopf A W, McCarthy
W H, Katz B. Surface microscopy of pigmented basal cell carcinoma. Archives
of Dermatology, 2000, 136(8): 1012-1016.
9. Braun R P, Rabinovitz H S, Krischer J, Kreusch J, Oliviero
M, Naldi L, Kopf A, Saurat J H. Dermoscopy of pigmented seborrheic keratosis:
a morphological study. Archives of dermatology, 2002, 138(12), 1556-1560.
10. Zaballos P, Malvehy J, Puig S. 5d Vascular lesions.
An Atlas of Dermoscopy, 2012. Informa Healthcare, London
11. Argenziano G, Catricalŕ C, Ardigo M, Buccini P, De
Simone P, Eibenschutz L, Ferrari A, Mariani G, Silipo V, Sperduti I, Zalaudek
I. (2011).Seven-point checklist of dermoscopy revisited. Br J Dermatol.
Apr;164(4):785-90.
12. Argenziano G. 6e Seven-point checklist and the seven
rules not to miss melanoma incognito. An Atlas of Dermoscopy, 2012. Informa
Healthcare, London.
13. Soyer H P, Argenziano G, Zalaudek I, Corona R, Sera
F, Talamini R, barbato F, Chimenti S. Three-point checklist of dermoscopy.
A new screening method for early detection of melanoma. Dermatology (Basel,
Switzerland), 2004, 208(1), 27-31.
14. Pehamberger H, Binder M, Steiner A, Wolff K. In vivo
epiluminescence microscopy: improvement of early diagnosis of melanoma.
Journal of Investigative Dermatology, 1993, 100: 356S-362S.
15. Kittler H. Dermatoscopy: introduction of a new algorithmic
method based on pattern analysis for diagnosis of pigmented skin lesions.
Dermatopathology: Practical & Conceptual, 2007, 13(1): 3.
16. Nachbar F, Stolz W, Merkle T, Cognetta A B, Vogt T,
Landthaler M, Peter B, Otto B F, Plewig G. The ABCD rule of dermatoscopy:
high prospective value in the diagnosis of doubtful melanocytic skin lesions.
Journal of the American Academy of Dermatology, 1994, 30(4): 551-559.
17. Menzies S W, Ingvar C, McCarthy W H. A sensitivity
and specificity analysis of the surface microscopy features of invasive
melanoma. Melanoma Research, 1996, 6(1), 55-62.
18. Rudnicka L,Olszewska M,Rakowska A.(2012). (eds.),Atlas
of trichoscopy, Springer, London.
19. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy:
a new tool for diagnosing skin infections and infestations. Dermatology,
2008, 216:14-23.
20. Teoli M, Di Stefani A, Botti E, Mio G, Chimenti S.
Dermoscopy for treatment monitoring of viral warts. Dermatology, 2006, 212:318.
21. Kim HO, Bae JM, Kim YY, et al. Differential diagnosis
of wart from callus and healed wart with aid of dermoscopy. Dermatology,
2006, 212:307.
22. Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of
molluscum contagiosum: a useful tool for clinical diagnosis in adulthood.
J Eur Acad Dermatol Venereol, 2006, 20:482-3.
23. Xavier MH, Ribeiro LH, Duarte H, Saraça G, Souza AC.
Dermatoscopy in the diagnosis of tinea nigra. Dermatol Online J, 2008, 14:15.
24. Brasiello M, Zalaudek I, Ferrara G, et al. Lupus vulgaris:
a new look at an old symptom: the lupoma observed with dermoscopy. Dermatology,
2008.
25. Pagliarello C, Fossati B, Landi F, et al. Dermoscopy
of a tick bite: a case report. Dermatology, 2006, 212:231.
26. Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis
capitis. J Am Acad Dermatol, 2007b, 57:727-728.
27. Argenziano G, Fabbrocini G, Delfino M. Epiluminescence
microscopy. a new approach to in vivo detection of Sarcoptes scabiei. Arch
Dermatol, 1997,133: 751-3.
28. Bakos RM, Bakos L. 'Whitish chains': a remarkable in
vivo dermoscopic finding of tungiasis. Br J Dermatol, 2008.
29. Tschandl P, Argenziano G, Bakos R, Gourhant J Y, Hofmann-Wellenhof
R, Kittler H, Zalaudek I. Dermoscopy and entomology (entomodermoscopy).
JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 2009, 7(7), 589-596.
30. 30. Vázquez-López F, Maldonado-Seral C, López-Escobar
M, Pérez-Oliva N. Dermoscopy of pigmented lichen planus lesions. Clin Exp
Dermatol, 2003a, 28:554–5.
31. Zalaudek I, Hofmann-Wellenhof R, Argenziano G. Dermoscopy
of clear-cell acanthoma differs from dermoscopy of psoriasis. Dermatology,
2003, 207:428.
32. Chuh AA. The use of digital epiluminescence dermatoscopy
to identify peripheral scaling in pityriasis rosea. Comput Med Imaging Graph,
2002, 26:129-34.
33. Zalaudek I, Lallas A, Moscarella E, Longo C, Soyer
H P, Argenziano G. The dermatologist's stethoscope-traditional and new application
of dermoscopy. Dermatol Pract Conc. 2013; 3 (2): 11.
34. Beltrán E, Toll A, Pros A, Carbonell J, Pujol RM. Assessment
of nailfold capillaroscopy by x 30 digital epiluminescence (dermoscopy)
in patients with Raynaud phenomenon. Br J Dermatol, 2007, 156:892–8.
35. Park JH, Lee DY, Cha HS, Koh EM. Handheld portable
digital dermoscopy: routine outpatient use for evaluating nail-fold capillary
changes in autoimmune connective tissue diseases. J Eur Acad Dermatol Venereol,
2008.
36. Baron M, Bell M, Bookman A, et al. Office capillaroscopy
in systemic sclerosis. Clin Rheumatol, 2007, 26:1268-74.
© 2015 Egyptian Dermatology Online Journal
|