Abstract
Background: Pityriasis rosea (PR), a common dermatological condition
of unknown etiology, affecting all ages. Aims: To study clinical and histopathological
features of PR. Methods: A total 50 patients from our outpatient department
with clinicopathological diagnosis of PR were studied over a period of 10
months from March 2014 to December 2014. In each patient, diagnosis was
made on clinico-pathological correlation. Various clinical and histopathological
features were analyzed. Results: The average age at onset of the disease
was 27 years, male: female ratio was 1.5: 1, and the average disease duration
was 2 months. Most cases were asymptomatic. Clinically, the most common
presentation was the classical PR with unusual features in 8 cases. Histologically,
no unusual features were seen except in two cases of purpuric and vesicular
PR. Conclusion: PR can present with variable clinical features. Histopathology
may help to achieve a correct diagnosis in doubtful cases.
Introduction
Pityriasis rosea (PR) is a common, benign skin condition. Exact etiology
is not known. A number of clinical variants have been classified. PR has
varied morphological presentations and may pose a diagnostic challenge in
some cases [1]. Skin biopsy is of vital
importance to make the correct diagnosis and treatment in such cases. We
studied 50 patients of PR over a period of 10 months.
Aim of the work
The aim of the present study was to characterize various clinical presentations
and histopathological variations of PR.
Patients and Methods
A total of 50 patients of PR from our outpatient department, with or
without any other skin disease were enrolled over a period of 10 months.
All the patients were subjected to detailed history, clinical examination
and skin biopsy after an informed consent. In each patient, the diagnosis
was made based on clinico-pathological correlation. Clinical variables,
such as age, sex, duration of disease, symptomatology, type of lesions,
family history, distribution of lesions, previous treatments taken if any
and previous clinical diagnosis were studied. Variables considered in histopathological
examination included epidermal changes, pattern of infiltrate, type of infiltrate,
vascular changes etc. Findings from all the patients were tabulated and
analyzed to characterize various clinicopathological features of PR
Results
Clinical
An average age of disease onset was 37 years. The youngest patient was
5 year old while the oldest was 54 year old. Males were more commonly affected
than females with a male female ratio of 1.5:1. An average duration of the
disease was 6 weeks with the shortest duration being 4 weeks while the longest
being 10 weeks. No recurrent PR was recorded.
No other major associated skin disease was seen in any of the patient.
No family history was noted in our study. Itching was the most common symptom
(30%), though the majority of the patients were asymptomatic (60%). No history
of drug intake was found in our study. Eighteen patients (36%) gave a history
of upper respiratory tract infection 2weeks before the onset of the lesions
while prodromal symptoms were found in 8 (16%) patients. History of Herald
patch (HP) was found in 28 (56%) patients and annular plaques with collaret
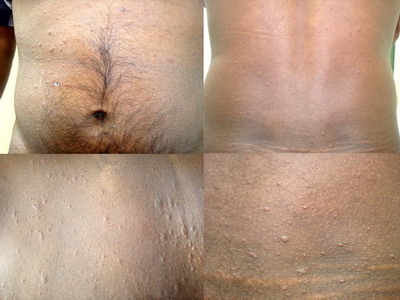
of scales were the most common type of lesions seen. The back was the most
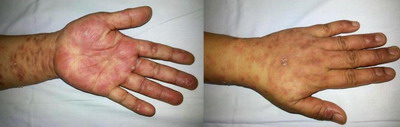
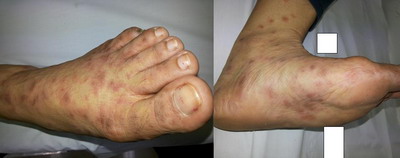
commonly affected site while involvement of the palms and soles was seen
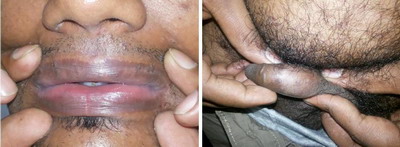
in 8 (16%) patients. (Figure 1& 2) Face was involved in 3 patients (6%)
(Figure 3), lips and prepucial skin in 1 patient each (2%) (Figure 4).
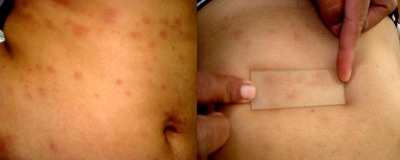
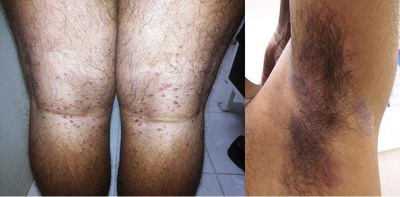
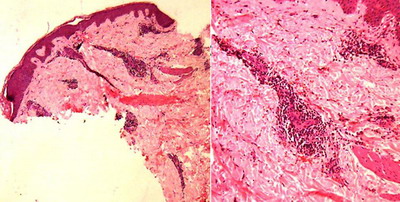
Purpuric PR (Figure 5) was seen in one case, clinically showing non-
blanchable lesions with Diascopy. Vesicular PR (Figure 6) was seen in one
case showing edematous papules and vesicles in typical PR pattern. Involvement
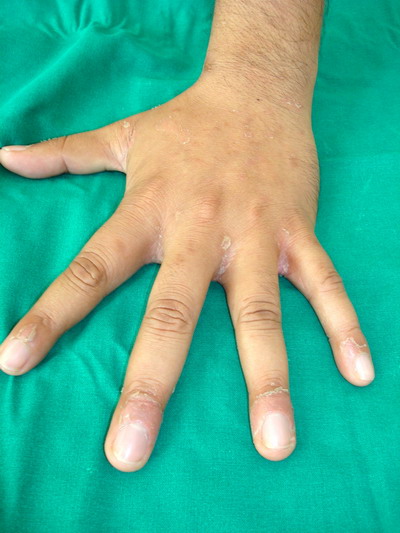
of the proximal nail fold and web spaces with typical collaret of scales
was seen in 6 cases. (Figure 7) while flexural involvement was seen in 3
cases. Two had annular lesion while one was papular PR. (Figure 8).
All the cases were treated with moisturizers, topical steroids and oral
antihistamines for itching. . Oral erythromycin 500mg qid was given in 15
cases and oral acyclovir 800mg tds was given in 20 cases while 15 cases
with milder disease were treated with only topical medicines. Short course
of oral steroids was given to only one patient with vesicular PR. Clinical
cure was almost the same in both groups treated with erythromycin and acyclovir.
Percentage of various types of PR and sites involved is shown in table
1.
|
Type of PR |
Number and Percentage |
Sites involved |
Number and Percentage |
|
Classic PR |
40 (80%) |
Back |
45 (90%) |
|
Papular PR |
5 (10%) |
Upper limbs |
42 (94%) |
|
Vesicular PR |
1 (2%) |
Lower limbs |
28 (56%) |
|
Purpuric PR |
1 (2%) |
Palms & soles |
8 (16%) |
|
Inverse PR |
3 (6%) |
Face |
3 (6%) |
|
Lichenoid |
0 (0%) |
Lips & prepuce |
1 (2%) |
Table-1: Percentage of various types of PR and various sites involved
Histopathological
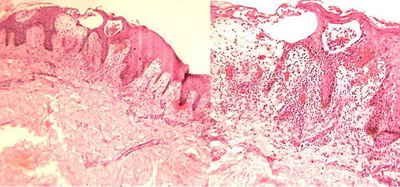
The majority of cases showed classical histopathological findings which
were sufficient to make diagnosis of PR on clinicopathological correlation.
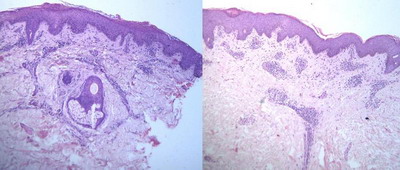
Parakeratosis with parakeratotic mounds, spongiosis, mild to moderate
acanthosis, superficial perivascular lymphocytic infiltrate with extravasation
of red blood cells (RBCs) and mild dermal edema were the most common findings.
(Figure 9).
Severe extravasation of RBCs was seen in purpuric PR (Figure 10) and
severe spongiosis with dermal edema was seen in vesicular PR (Figure 11).
Various histopathological findings with their percentage are listed in
table 2.
|
Histopathological feature |
Percentage of cases |
|
Parakeratosis |
100% |
|
Parakeratotic mounds |
80% |
|
acanthosis |
90% |
|
spongiosis |
80% |
|
Lymphocytic exocytosis |
70% |
|
Superficial perivascular infiltrate |
95% |
|
Papillary dermal edema |
90% |
|
Red cell extravasation |
80% |
|
Eosinophils infiltrate |
20% |
|
Dyskeratotic cells |
20% |
|
Multinucleate cells |
0% |
|
Apoptotic cells |
0% |
|
Acantholytic dyskeratosis |
0% |
Table-2: Percentage of various histopathological findings
We regret that owing to constraints in resources, we could not study
any hematological parameters and immunohistochemical markers.
Discussion
The PR is a self-limiting disease with sudden-onset and with specific
skin rash and usually affects the children and adolescents [1].
PR does not have any predilection for sex and equally affects both of
the genders [2]. However in our study males
were affected more than females with the male-female ratio of 1.5/1. PR
is known to have seasonal variation with high number of cases found in winter
season probably because of increased incidence of throat infection at the
same time. It may become epidemic in crowded living spaces such as school,
family and workplaces [3]. History of throat
infection was noted in 36% of patients in our study. History of throat infection
was noted in 17.5% of patients in a study by Sharma et al [4].
Ozyurek et al., found throat infection in almost an equal male female ratio
[5], but in many patients it may not be
noted or may be subclinical.
The PR is sometimes observed in the areas of insect bite, minor skin
infection, previous scars, injection sites or BCG vaccination, new, unwashed
clothes or clothes held dirty for a long time suggested to be associated
with the disease [4,6].
No such observation was made in our study.
Prodromal symptoms are usually rare but occasionally findings such as
fever, headache, throat pain, abdominal pain, arthralgia, cough, vomiting
and lymphadenopathy may be observed. Only 8 patients (16%) reported prodromal
symptoms just before the onset of the rash in our study. Body aches and
throat pain with mild hyperthermia were the most common prodromal symptoms.
Ozyurek et al., reported a frequency of prodromal symptoms of 59.6% of patients
in their study and suggested that the higher rate supports the role of viral
etiology of PR [5].
Frequency of HP ranges between 40% and 76% amongst various studies. It
was 56% in our study. A study by Ozyurek et al., found it in 77% of patients
[4,6]. The
HP lesion were reddish brown with slight peeling of the skin. The most common
site was the back followed by the abdomen, the chest and the extremities.
The lesions were round or oval in shape, and the size varied from 2 to 6
cm. Secondary eruptions occurred within 10 days after HP in 62.5% of the
patients in the study of Sharma et al. [4]
and 87.5% of the patients in study by Ozyurek et al., [5]
while it was reported in 72% of the patients in our study. We did not found
multiple HPs in our study. Sharma et al found up to 3 HPs in some patients
[4].
Itching was the most common symptom in our study. It was moderate in
the majority of cases and very few patients needed antihistamines. None
of patient reported any aggravating factor for itching. Sharma et al., found
sunlight as one of the triggers for itching in their study [6].
Cases were maximum during the 4 months from September to December; and
minimum from March to July. The highest number was seen in October (14 cases
out of 50 cases). Similar seasonal variation has been observed by sharma
et al., and Ayanlowo O et al., in their studies [4,7].
None of our patients gave a history of similar eruption in past and no
recurrent PR was found in 6 months follow up in any patient. Sharma et al.,
found a past history of eruptions similar to those of PR in 5 patients with
the longest duration of 2 years [4]. None
of our patients had history of any other skin disease, though some studies
have reported history of atopy in some patients. Regarding the family history
of PR, our finding were similar to that of study by sharma et al., with
no family history of similar lesions [4].
The distribution of lesions was bilateral and almost symmetrical with
long axis along the cleavage lines in all cases except one case showing
predominantly left sided lesions. The trunk was the commonest site followed
by extremities. Inverse type lesions were seen in five patients with predominant
flexural involvement. No giant PR was seen. Palmo-plantar involvement was
seen in 8 (16%), face in 3 (3%) and mucosal involvement (lips and prepucial
skin) in 1 patient.
The size of the secondary eruptions lesions varied from 0.5 to 4 cm.
Most were slightly erythematous to light brown, multiple, discrete, oval
(96%) plaques with fine and dry scales in center and collaret at the periphery.
The lesions were papular in 1 patient and vesicular in one. Follicular or
targetoid lesions were not seen in our study while Sharma et al., found
them in 1 patient each [4].
Histopathological features of PR are not specific and findings are variable
depending on the stage of the lesions in evolution. In this study we tried
to find some common and consistent histopathological features of PR. Different
studies have shown different findings ranging from eczematous change in
early lesion to parapsoriasis en palque-like changes in late lesions [4,5,8].
Parakeratosis was the most consistent finding in our study with 100%
occurrence, followed by superficial lymphocytic infiltrate (95%). Okamoto
et al. conducted a study in 29 patients with PR, and found perivascular
lymphocytic infiltration in all biopsy materials followed by erythrocyte
extravasation in 66% of biopsy materials [9].
We found erythrocyte extravasation in 80% of the biopsies.
Acanthosis was seen in 90% of cases, papillary dermal edema in 90% of
cases, Spongiosis was seen in 80% of cases while lymphocyitc exocytosis
in 70% of cases in our study. These findings are comparable with a study
by Panizzon et al., who evaluated 62 biopsies and the main characteristic
feature found was eczematous manifestation [10].
Similar rates were reported in a study by Aiba et al., but the study
sample was too small [8]. Ozyurek et al.,
have debated about role of cellular immunity in pathogenesis of PR [4]
based on features like exocytosis of lymphocytes, dyskeratotic cells in
significant number of cases suggests a possible interaction between epidermal
components and dermal mononuclear cells in the pathogenesis of PR. Also
dyskeratotic cells signify basal layer damage. Morever, erythrocyte extravasation
in large number of biopsies in most of the studies supports the concept
of dermal vascular damage [4,7,8,9].
In our study, also lymphocytic exocytosis, dyskeratotic cells and erythrocyte
extravasation along with perivascular lymphocytic infiltrate and dermal
edema were seen in most cases and we also feel that cellular immunity is
of importance in pathogenesis of PR.
In our study, severe extravasation of erythrocytes with clinically purpuric
lesions was seen in one case. Also severe spongiosis and exocytosis with
intra-epidermal vesicles with vesicular lesion clinically was seen in one
case.
We could not do any immunohistochemical markers due to economic constraints
but many studies have proven the inflammatory infiltrate to be of T cell
origin with positive staining for CD3 marker and negative for CD20, a marker
for B cell. [4,7,9,10].
These findings supports the speculation that PR is associated with cellular
immunity rather than humoral immunity.
In our study, observation of perivascular lymphocytic infiltration, lymphocytic
exocytosis, focal parakeratosis, parakeratotic mounds, irregular acanthosis
and spongiosis and superficial dermal edema in more than half of our biopsy
materials appear to support the eczema-like histopathologic features of
PR.
We suggest that the above mentioned histopathological features can be
used reliably for diagnosis of PR in presence of clinical criteria given
by Zawar et al [11].
Conclusion
PR is a mild eczematous dermatosis, occurring in winter mostly, probably
triggered by infection and remitting within around 8 weeks.
References
1. Sterling JC, Burns T, Breathnach S, et al. Virus infections.
Rook's Textbook of Dermatology. 2004; 25: 79-83
2. Tay YK, Goh CL. One-year review of pityriasis rosea at
the National Skin centre, Singapore. Ann Acad Med Singapore. 1999; 28: 829-31
3. Chuang TY, Perry HO, Ilstrup DM, Kurland LT. Recent upper
respiratory tract infection and pityriasis rosea: A case control study of
249 matched pairs. Br J Dermatol 1983; 108: 587-91
4. Sharma L, Srivastava K. Clinicoepidemiological study
of pityriasis rosea. Indian J Dermatol Venereol Leprol 2008; 74: 647-9
5. Ozyurek GD, Alan S, Cenesizoglu E. Evaluation of clinico-epidemiological
and histopathological features of pityriasis rosea. Postepy Dermatol Alergol.
2014; 31(4): 216-221.
6. Slebioda Z, Szponar E, Kowalska A. Recurrent aphthous
stomatitis: genetic aspects of etiology. Postep Derm Alergol. 2013; 30:
96-102.
7. Ayanlowo O, Akinkugbe A, Olumide Y. The pityriasis rosea
calendar: a 7 year review of seasonal variation, age and sex distribution.
Nig Q J Hosp Med 2010; 20(1): 29-31.
8. Aiba S, Tagami H. Immunohistologic studies in pityriasis
rosea. Evidence for cellular immune reaction in the lesional epidermis.
Arch Dermatol. 1985; 121: 761-5.
9. Okamoto H, Imamura S, Aoshima T, et al. Dyskeratotic
degeneration of epidermal cells in pityriasis rosea: light and electron
microscopic studies. Br J Dermatol. 1982; 107: 189-94
10. Panizzon R, Bloch PH. Histopathology of pityriasis
rosea Gibert: qualitative and quantitative light microscopic study of 62
biopsies of 40 patients. Dermatologica. 1982; 165: 551- 8.
11. Zawar V, Chuh A. Applicability of proposed diagnostic
criteria of pityriasis rosea: Results of a prospective case-control study
in India. Indian J Dermatol 2013; 58: 439- 42.
© 2015 Egyptian Dermatology Online Journal
|