|
|
Abstract
Integrins are heterodimeric transmembrane
glycoprotein composed of non-covalently linked a
and b subunits. They are cell surface
adhesion molecules essential for cell-matrix and cell-cell interaction and are
capable of transmitting signals from within or from without the cell.
Integrins are important for epidermo-dermal
anchorage. Genetic or autoimmune dysfunction of a6b4
results in blistering of skin and mucous membranes, like junctional
epidermolysis bullosa with pyloric atresia and cicatricial pemphigoid
respectively. Expression of different types of integrins on the surface of
keratinocytes, fibroblasts, endothelial cells and blood cells is essential for
wound healing.
In malignancy, increased expression of integrin
promotes tumor growth and metastasis. Metastasis is promoted by integrins in
three principle mechanisms, through stimulating cell migration, production of
proteases and by increasing blood vessel formation.
Beta 2 integrins on leukocytes are important
for their recruitment to sites of inflammation and infection. Genetic
dysfunction of b2 subunit results in the immune deficiency syndrome known as
leukocyte adhesion deficiency, characterized by inability of leukocytes to
migrate and phagocytose. Beta 2 integrins also help in providing close contact
for antigen presentation to T-lymphocytes and leukocyte activation. LFA-1/
ICAM-1 interaction between T-lymphocytes and somatic cells is seen in many
immunologic skin conditions, such as contact dermatitis, psoriasis, lichen
planus, vitiligo, scleroderma and others, which make them potential drug
targets.
Introduction
Hynes in 1987[1] was the first to apply
the term "Integrin" to describe a family of structurally,
immunochemically and functionally related receptors, which integrated
the extracellular matrix with the intracellular cytoskeleton to mediate cell
adhesion and migration.
Integrins are molecules essential for cell-cell
and cell-substrate adhesion. They are cell surface transmembrane glycoproteins
that function as adhesion receptors transmitting biochemical and mechanical
signals in a bidirectional manner across the plasma membrane and thus influence
most cellular functions such as proliferation, differentiation, apoptosis and
cell migration [2, 3]. Among a host of other adhesion molecules,
including selectins, members of immunoglobulin superfamily, and cadherins,
together, they participate in every cellular activity, although they may be
latent, i.e. not expressed until the cell is stimulated, expressed very
transiently or may be expressed for longer periods [4].
Integrins and their ligands play key roles in
development, immune responses, leukocyte trafficking, and hemostasis. They share
in the pathogenesis of many genetic, autoimmune and malignant diseases, and are
the receptors for many viruses and bacteria. Hence, integrins are a promised
target of future effective drugs [2, 5] .
A brief discussion of integrin structure,
variable modes of action, important role in cutaneous health and disease, and
their future role as drug targets will be presented.
Integrin
Structure & Mechanism of Action
Structure:
Each integrin is a heterodimer
composed of an alpha (a) and a beta (b)
subunit that are stabilized by non-covalent bonds. On searching the human
genome, 18 a and 8 b
subunits have been recognized. Some limitations for dimer formation using
certain a and b subunits exist resulting in the formation of only 24 different
integrins [2, 6].
Although there are no genetic relations between
a- and
b-subunits, they share similarity in domain structure
[7]. Each
subunit contains three domains: a big glycosylated extracellular domain
(consisting of more than 90% of the whole molecule), a hydrophobic transmembrane
domain (responsible for membrane anchoring) and a small cytoplasmic domain
(.Figure 1). The size of
a-subunits
varies from 120-180kD, while that of b-subunits
varies from 95-117 kD (8, 9].
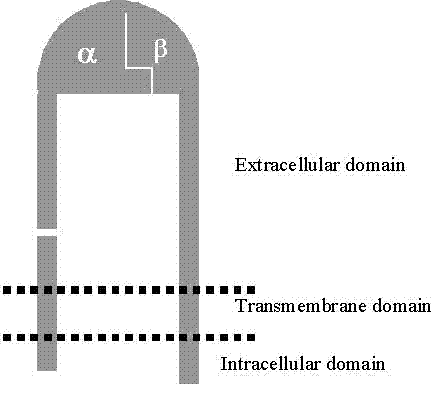
|
Figure 1 Integrin Structure, showing large extracellular domain and small cytoplasmic domain
(20-50 amino acids for a- and 15-65 amino acids for b-subunits) The a-subunit complexes with b-subunit to form the ligand-binding
"head", which is attached to two "legs", one from
each subunit. The cytoplasmic domain is also referred to as the “tail” Modified
from Berman et al., 2003[2] |
Both subunits
are required for ligand binding. This is consistent with the fact, that
switching either subunit can change receptor specificity. Individual integrins
can bind more than one ligand, and similarly, individual ligands are often
recognized by more than one integrin so there is no absolute specificity (Table 1). This is related to the ability of integrins to recognize and bind to small
specific oligopeptide sequences, such as RGD sequence (Arg-Gly-Asp) present on a
number of ligands as fibronectin, vitronectin and other adhesion proteins
[10]. Furthermore, the cytoplasmic domains play a primary role in ligand and
signal properties of integrins and variability of these sites is a basis for the
diverse functions of the whole integrin family and unique functions of certain
receptors [6]. Many integrins are not constitutively active; they are
in an “OFF" state, in which they do not bind ligands and do not signal. Integrin
activation can occur from outside after
binding with its specific ligand "outside-in"
signaling, or it can occur from inside "inside-out" signaling [11, 12].
Mechanisms of function of integrins
Cell Anchorage:
Integrins were first known to be involved in cell anchorage to the extracellular
matrix (ECM), linking it to the cytoskeleton [1]. The extracellular
domain binds to ECM protein and the cytoplasmic domain links to cytoskeletal
actin microfilaments. Certain linker proteins play an intermediate role in this
binding activity, like talin and vinculin [10].
Integrins tend to be present in clusters like
focal adhesions, which further serve to strengthen their attachment to the ECM
and to concentrate messengers transducing signals from integrins and other cell
receptors to the genome [2].
Signal Transduction:
The second important function of integrins is
their role in signal transduction. Both integrin subunits are needed for signal
transduction. However, the
b-subunit
appears to have a more superior role, since most of cytoplasmic proteins bind to
it [13, 14]. Stimulation of different signaling pathways depends on the
type of integrin, type of ECM attached, and other diverse internal and external
stimuli. External factors that stimulate integrins include cytokines, growth
factors, chemokines, and adhesion molecules including other integrins[15] .
Integrins are involved in many aspects of cell
behavior including proliferation, shape, polarity, differentiation, migration,
and survival/apoptosis. Integrins mediate these functions through complex
signaling pathways in which kinases, G-proteins, adapter proteins and
proto-oncoproteins are involved [2]. The role of integrins in
cell proliferation represents a good example illustrated in [16, 17].

|
Figure 2.
Epidermal Basement
Membrane showing its molecular components (courtesy of MA Abdallah) |
Role of
integrins in development
Knockout mice for different integrin subunits
show a different phenotype. The phenotypes range from complete block in
preimplantation development, through major developmental defects and
perinatal lethality to less severe functional defects [6]. Knockout
of certain integrins that potentially bind to the same ligand, shows the
relative role of each one, underscoring the similarities and differences in
functioning. For example, mice lacking the gene for
a1
and hence deficient in the collagen binding integrin
a1
b1
maintained viability and fertility, but showed dermal hypoplasia and
reduction of proliferation activity. However mouse embryos deficient in
a2 subunit involved in the other collagen binding integrin
a2
b1 die at the stage of implantation [2].
Role of
integrins in proliferation, differentiation and apoptosis
Most normal cells do not divide when present
in suspension; they need to be anchored to a solid substrate. This is
mediated via integrins, which provide both binding and transduction of
essential signals. For epidermal differentiation to occur, a downregulation of
the laminin binding integrins
a6b4 and a3b1
integrins is demonstrated [18, 19]. Cell anchorage also seems
inhibitory to cellular apoptosis, integrin binding regulate apoptosis by
their bifunctional capability in anchorage and signaling [20].
Keratinocyte Integrins
Keratinocytes exhibit a
number of integrins, which are essential for their anchorage and migration. Some
of them are constitutively expressed, while others are expressed or upregulated
upon stimulation by wounding, pathological changes, or in culture. The most
abundant constitutive integrins in the epidermis are
a6b4, a2b1
and a3b1.
Alpha v beta 5 is also a constitutive epidermal integrin, but is expressed at
lower levels than the others. Alpha5 beta1 and
avb6
are induced in culture and on wounding, while
a9b1
is expressed in low concentration in undamaged epidermis and is upregulated
during wound healing. Two other integrins have been found in epidermis;
a8b1, which is confined to the arrector pili muscle and
avb8, which is the only integrin found in normal suprabasal epidermis[21] (Table1).
Integrins of the normal
undamaged epidermis are confined to the basal layer and outer root sheath of the
hair follicle. Alpha 6 beta 4 is confined to hemidesmosomes, while other
integrins are distributed over the basal, lateral, and apical surfaces of basal
cells. Beta 1 integrins in the basal aspect of basal cells are present in
clusters interspersed with hemidesmosomes, but the majority of
b1
integrins appear to form an 'O' ring at the cell periphery [20].
Alpha6 Beta4 Integrin
Alpha6 Beta4 Integrin and
Hemidesmosomes
In normal human
keratinocytes, a6 and b4 subunits combine exclusively with each other. The integrin
a6b4 is restricted to the basal surface of resting epidermal cells confined
to hemidesmosomes. Other components of the hemidesmosomes include plectin,
bullous pemphigoid antigen type 1 (BPAG1, 230 kD), bullous pemphigoid antigen
type 2 (BPAG2, 180 kD) and CD151 tetraspanin [8, 22] (figure 2).
The general structure of
a6b4
is the same as for other integrins. The exception lies in the big cytoplasmic
portion of the b4
subunit (about 1000 amino acids), which binds keratin intermediate filaments
instead of actin. Absence of this big cytoplasmic tail prevents hemidesmosomal
assembly. The membrane proximal region of the cytoplasmic domain of the
b
subunit directly associates with plectin, while its distal region binds BPAG2
and BPAG1 [21]. The proximal extracellular domain of the
a subunit binds a region on BPAG2, and is responsible for localizing it
to hemidesmosome [5, 23].
Laminin 5 and laminin 1 are
the preferred extracellular ligands for
a6b4 [24]. Laminin 5 (epiligrin,
kalinin) is the major laminin in epidermal
basement membrane (figure 2). Both laminin 5 and
a6b4
integrin are localized to anchoring filaments, where laminin 5 further links
integrin directly to type VII collagen of anchoring fibrils and indirectly to
other components of the lamina densa[18]. Integrin
a6b4 and laminin 5 play a crucial role in the proper assembly of
hemidesmosomes, as deficiency of either protein leads to impaired hemidesmosome
formation. Alpha 6 beta 4 integrin is also important in stabilization of the
dermal-epidermal junction by connecting the intermediate filaments with the ECM
[25].
Alpha6 Beta4 Integrin and
Cell Migration
Although
a6b4
is primarily responsible for cell anchorage, it was found to be involved in cell
migration and invasion,
which are important for wound healing and tumor
progression. It has been detected on the leading edge of migrating keratinocytes
in association with filamentous actin [26]. The role of
a6b4
in migration and invasion implies changes in mechanical [25, 27],
as well as signaling properties of the integrin [28, 29], which are
combined with structural changes of laminin 5 as well as changes in the
localization of laminin 1 and 5 [25, 30]. Migration could be mediated
by growth and chemotactic factors [29].
Diseases due to
malfunction of Alpha6 Beta4 Integrin
Junctional Epidermolysis Bullosa with Pyloric
Atresia
Junctional epidermolysis bullosa with pyloric
atresia (JEB-PA) is an autosomal recessive disorder resulting from mutations in
genes encoding either
a6
[31] or b4
integrin subunits [32]. Clinically, it is characterized by mucocutaneous
fragility and gastrointestinal atresia, which most commonly affects the pylorus.
Additional features of JEB-PA include involvement of the urogenital tract,
aplasia cutis, and failure to thrive. While most affected individuals have a
poor prognosis resulting in death in infancy (lethal type), others have milder
clinical features and a better prognosis (non-lethal type) [33].
Absence of either integrin subunit results in
ultrastrucurally abnormal hemidesmosomes with absent or decreased immunostaining
of the respective, or even both integrin subunits at the basement membrane zone
[31, 32, 34].
Cicatricial Pemphigoid
Cicatricial pemphigoid is a heterogeneous group
of autoimmune subepidermal blistering diseases characterized by a predominant
involvement of the external mucosal surfaces and a tendency to scarring. It is
associated most commonly with autoantibodies to bullous pemphigoid 2 (BPAG2) and
less frequently with those to laminin 5 or type VII collagen. In addition, a few
cases have been described with autoantibodies to the
b4 integrin subunit. These patients present with predominant ocular
involvement [35].
Alpha 3 beta 1
The integrin
a3b1 is predominantly expressed in basal keratinocytes, between
hemidesmosomes as well as laterally, where it shares in epidermal-dermal
cohesion [36]. It binds primarily to laminins, especially laminin 5,
and in certain situations it can bind to collagen and fibronectin. When laminin
5 of resting keratinocytes binds
a3b1, it inhibits keratinocyte migration. Their binding also regulates
cellular proliferation and differentiation [23]. During wound healing,
however, laminin 5 promotes keratinocyte migration. This occurs secondary to its
cleavage by matrix metalloproteinase 2 (MMP-2, a gelatinase) released from
activated epithelial cells [37].
Alpha2 beta 1
Alpha 2 beta 1 is a collagen receptor expressed
by keratinocytes in abundance. It mediates keratinocyte migration over collagen
during cutaneous wound repair. Epidermal growth factor (EGF) and transforming
growth factor a
(TGFa) upregulate
a2b1
expression, further enhancing their motility [38]. Once
a2b1 binds type I collagen, epidermal cells respond by producing
collagenase (MMP-1). This enzyme is required for epidermal migration over
collagen. [39]. In migrating cells,
a2b1 moves from the lateral aspect of the cell to the very tip of the
migrating edge [5].
Alpha 5 beta 1
Alpha 5 beta 1 is the classic fibronectin
receptor that is expressed by keratinocytes only during wound repair. This
integrin appears to be involved in keratinocyte motility [40].
Fibronectin is a multifunctional protein that is deposited early during wound
healing as a part of the provisional matrix. Fibronectin together with TGFb
induce the expression of
a5b1 on keratinocytes, making them capable of migration on the provisional
matrix [41]. When activated by fibronectin fragments,
a5b1, in its turn, stimulates the synthesis of collagenase (MMP-1),
stromelysin (MMP-3) and gelatinase (MMP-9) [42].
Alpha v integrins
Vitronectin is one of the provisional matrix
proteins deposited at sites of injury. In most cells, the only integrins that
bind to vitronectin contain an
av
subunit [11]. Epidermal cells express
avb5 as they migrate over provisional matrix containing vitronectin during
re-epithelialization of cutaneous wounds [41].
Another
av
receptor expressed by wound epidermis is
avb6. It is a tenascin and fibronectin receptor rather than a vitronectin
receptor [23]. During the first few days of migration the basal
epidermal cells express
avb5,
while avb6 is present around the time of fusion. This timed expression is related
to the presence of vitronectin early and tenascin later during healing [43].
Integrins and Wound Healing
Wound healing is a complex, ordered process,
which occurs after tissue injury, in an attempt of the body to regain its
integrity. Keratinocytes, fibroblasts, endothelial cells and blood components
are involved in wound healing. Integrin receptors are required for all phases of
wound repair [5]. All steps follow a specific time sequence, and can be
temporally classified into inflammation, tissue formation, and tissue
remodeling. These phases of wound repair, however, are not mutually exclusive
but rather overlapping in time [44].
Inflammation
Injury to blood vessels stimulates intrinsic and
extrinsic coagulation cascades. Successful hemostasis depends on platelet
adhesion and aggregation. Platelets also release mediators and adhesive proteins
including fibrinogen, fibronectin, thrombospondin and von Willebrand factor. The
first three act as ligands for platelet aggregation, while von Willebrand factor
mediates platelet adhesion to fibrillary collagens [45]. Platelet
adhesion to all four proteins is mediated through
aIIbb3 and other surface integrins. Alpha IIb beta3 also mediates platelet
driven clot retraction [46] .
Neutrophils followed by monocytes begin to
emigrate into injured tissue. Both types of cells are recruited by chemotactic
factors released in the injured area and their migration is facilitated by
b2 integrins (CD11/CD18 and other surface proteins [47].
Infiltrating neutrophils attempt to clear the area of foreign particles,
including bacteria. Effete neutrophils in the wound are either extruded with the
eschar or phagocytosed by macrophages or fibroblasts [44].
Once in tissue, monocytes progressively activate
and phenotypically become macrophages. This occurs secondary to integrin binding
to the ECM, which also stimulates signal transduction followed by the secretion
of cytokines such as colony stimulating factor, tumor necrosis factor a (TNFa), and platelet-derived growth factor (PDGF). Such growth factors are
necessary for initiation and propagation of new tissue formation in wounds [48].
Binding of monocytes or macrophages to specific
ECM proteins through integrin receptors also stimulates ECM phagocytosis and Fc-
and C3b-mediated phagocytosis. Thereby macrophages are armed to debride tissue
through phagocytosis and digestion of pathogenic organisms, tissue debris, and
effete neutrophils [5, 44].
Tissue Formation
Re-establishing a cutaneous cover
Basal keratinocytes from residual epithelial
structures undergo morphological changes, lose their firm attachment with the
basement membrane, and attain a migratory phenotype expressing migration-related
integrins [5, 44]. The migrating wound epidermis does not simply
transit over a wound coated with provisional matrix but rather dissects through
the wound, separating desiccated or otherwise nonviable tissue from viable
tissue [49]. The path of dissection appears to be determined by the
array of integrins that the migrating epidermal cells express on their cell
membranes. Since avb3, the receptor for fibrinogen/fibrin and denatured collagen, is not
expressed on keratinocytes, keratinocytes do not have the capacity to bind to
this matrix protein [50, 51]. Hence the migrating wound epidermis
avoids the fibrin-rich clot and migrates over dermal type I collagen permeated
with fibronectin, vitronectin and tenascin, utilizing
a2b1 collagen receptors,
a5b1
fibronectin receptors,
avb5 vitronectin receptors, and
avb6 tenascin receptors, respectively [41].
As mentioned earlier,
a6b4 and a3b1
contribute to cell migration over laminin after inducing structural changes
within laminin [26, 37]. Migrating epidermal cells secrete plasminogen
and plasminogen activator [39], in addition to MMPs secreted on
integrin binding with their ECM ligands, in order to dissect their path through
the provisional matrix [42].
Re-establishing a Dermal Integrity
Migrating monocytes, fibroblasts and newly
forming blood vessels use integrin receptors that recognize provisional matrix,
which is composed of fibrin, fibronectin, and vitronectin. Moreover, binding of
ECM to integrin receptors provides signals for gene expression necessary to
modulate cellular functions and secrete substances essential for wound healing [11, 44]. PDGF, TGFa and TGFb act in concert with each other to stimulate fibroblast proliferation,
migration into the wound and later protein synthesis and wound retraction to
occur [52].
PDGF stimulates the expression of different
integrins on fibroblasts according to the type of the extracellular matrix
environment, thereby promoting cell migration [12, 52, 53]. In
addition, integrin binding to ECM stimulates the secretion of proteases to help
fibroblasts dissect their path through the blood clot [44].
Once the fibroblasts have migrated into the
wound, they start depositing loose ECM, composed of great quantities of
fibronectin, followed by the deposition of abundant fibrillar collagen I, III
and VI. As soon as a collagen matrix is deposited, fibroblasts remodel it by
wound contraction. This occurs when fibroblasts assume a myofibroblast phenotype
[54]. Fibroblasts link to the extracellular fibronectin matrix mainly
through a5b1 to collagen matrix through
a1b1 and a2b1
collagen receptors; and to each other through direct adherens junctions [55].
New collagen bundles join end-to-end with collagen bundles at the wound edge.
These links provide a network across the wound whereby the traction of
fibroblasts on their pericellular matrix can be transmitted across the wound [5].
Neovascularization
During wound healing, angiogenic capillary
sprouts invade the fibrin/fibronectin-rich wound clot and within a few days
organize into a microvascular network throughout the granulation tissue.
Endothelial cell migration and capillary formation is mediated by the binding of
avb3
expressed on their surface [56, 57].
Tissue
Remodelling (Transition from provisional matrix to collagenous scar)
Endothelial cells followed by myofibroblasts and
macrophages undergo programmed cell death (apoptosis), ultimately leading to a
rather acellular scar. Over the ensuing weeks, fibronectin and hyaluronic acid
disappear; collagen bundles grow in size, increasing wound tensile strength; and
proteoglycans are deposited, increasing wound resilience to deformation [44].
Integrins and Inflammation/ Immunity
Integrins important in the interaction of
leukocytes with the endothelium fall into three groups: Beta2 integrin family
(designated CD11/CD18) is composed of
aLb2 (CD11a/CD18, leukocyte function associated antigen-1, LFA-1),
aMb2 (CD11b/CD18, Mac-1),
aXb2
(CD11c/CD18, p150, 95) and
aDb2
(CD11d/CD18)[4, 47]. These four integrins demonstrate some sort of
cell type and ligand specificity. While the first three are distributed on
neutrophils and monocytes, B- and T-lymphocytes express only LFA-1, and
eosinophils express p alone. Similarly, they share some but not all ligands.
LFA-1 binds to all three members of the ICAM family (ICAM-1,2,3), Mac-1 binds
only to ICAM-1, but additionally binds complement receptor iC3b (CR3),
fibrinogen and factor X, binds to iC3b and fibrinogen, while CD11d/CD18 binds
preferentially to ICAM-3 [58]. ICAM-1 expression is induced on
endothelial cells and other cells by inflammatory cytokines, but ICAM-2 is
constitutively expressed and therefore may be more important in lymphocyte
recirculation that occurs in uninflamed skin [5].
Two
a
4 integrins are important in leukocyte/endothelial cell interactions:
a4b1
(very late antigen-4, VLA-4) and
a4b7.
While both of these integrins are present on B- and T-lymphocytes, VLA-4 is also
expressed on monocytes and eosinophils. It binds to vascular cell adhesion
molecule-1 (VCAM-1). VCAM-1 is expressed on the endothelial cells by cytokines [5]. VLA-4 binds also to fibronectin. Alpha 4 beta 7 binds mainly to
the mucosal addressin cell adhesion molecule (MAdCAM) in addition to its
capability to bind to VCAM-1 and fibronectin (Table 1) [47].
Lastly,
aEb7 integrin is expressed on lymphocytes [47] and binds
E-cadherin on epithelial cells [10].
Role of integrins in inflammation
Leukocytes flow in the middle of the blood
stream. When they are needed; they approach the blood vessel margin under the
effect of shear
forces [4]. This is followed by tethering and rolling, arrest,
adherence and lastly emigration of leukocytes through the blood vessel wall
These events occur mostly in postcapillary venules, owing to their high
expression of adhesion molecules [4, 5] and could be summarized as
follows:
Tethering and rolling
Tethering is mainly mediated by selectins [47].
L-selectin on leukocytes binds to its receptors (CD34 and
glycosylation-dependent cell adhesion molecule-1 [GlyCAM-1]) on endothelial
surface, followed by cleavage and L-selectin shedding [59]. Concomitant
with the increased avidity of L-selectin is increased expression of
b2 integrin, which at this stage is not yet activated. P-selectin of
endothelial and plasma cells is rapidly mobilized to the plasma membrane and
binds transiently to neutrophils and monocytes, contributing to their rolling [4, 60]. E-selectin is produced under the effect of IL-1, TNFa,
lipopolysaccharide, substance P and mast cell degranulation on the surface of
endothelial cells [61]. E-selectin binds to cutaneous leukocyte antigen
(CLA) on lymphocytes and to specific carbohydrate residues on neutrophils [47].
Arrest and adhesion
Integrins of mobile, non-activated leukocytes do
not bind to endothelial cell ligands, thus avoiding spontaneous cell aggregation
and vascular damage. The classical chemoattractants (C5a, platelet activating
factor, leukotriene B4 and N-fromyl peptides) stimulate neutrophils, monocytes
and eosinophils with minimal cell type specificity. Chemokines, which are
divided into two subfamilies, CXC and CC show more specificity. CXC chemokines
like IL-8 act on neutrophils and fibroblasts involved in wound healing; while CC
chemokines like MCP (monocyte chemotactic proteins) act on monocytes,
eosinophils and lymphocyte subpopulations [58].
Binding of chemoattractants to specific leukocyte
transmembrane receptors activates adhesiveness of integrin receptors on the
surface, the second step in the process of recruitment [5]. LFA-1 and
Mac-1 show conformational changes and increase in ligand binding sites after
activation of the cell with resultant binding to their endothelial ligands
(Table1). Leukocyte arrest at the site is followed by emigration or local
inflammatory change. Activation of LFA-1 by binding to ICAM-1 stimulates a
number of phosphorylation events, which further stabilize leukocyte adhesion to
endothelium. Activation of integrins also stimulates cell synthesis of cytokines
and receptors to chemokines contributing to cell emigration [79].
Emigration
Leukocytes leave vessels through
inter-endothelial cell junction. The molecular mechanisms of leukocyte
transmigration involve CD31, which is expressed both by leukocytes and
endothelial cells, and interacts with itself. CD31 of activated lymphocytes
could displace CD31-CD31 homophilic binding at endothelial junctions [47].
Role of integrins in cutaneous immunity
LFA-1 in antigen presentation
Antigen processing and presentation by antigen
presenting cells (APC), like Langerhans cells, to T-lymphocytes is a
prerequisite for T-cell activation. Antigen is presented in conjunction with
class I or II major histocompatibility complex (MHC-I or II) to T-cell receptor
(TCR), which is complexed with CD3 (TCR/ CD3 complex). TCR recognition of
antigen bound to MHC is insufficient to lead to T-cell proliferation or effector
function. There is a requirement for additional "co-stimulatory" signals. This
involves close cell-cell contact mediated by several different adhesion
molecules on either cell in addition to stimulatory cytokines, notably IL-1 and
IL-2. Among the recognized adhesion molecules is binding of LFA-1 on T-cells to
ICAM-1 on APC. The classical example of APC/ T-cell interaction is the afferent
phase of allergic contact dermatitis, which demonstrates increased ICAM-1
expression on Langerhans cells as well as on keratinocytes [4].
Role of integrins
in some dermatoses
Beside antigen presentation, interaction between
ICAM-1 and its ligand LFA-1 is a necessary step for contact-dependant
immunologic reactions via leukocytes [63]. LFA-1 expression is
increased on lymphocytes and ICAM-1 is expressed on vascular endothelial cells
at sites of inflammation as well as on keratinocytes, fibroblasts or melanocytes
in a number of immunologically mediated skin conditions. In psoriasis [64],
lichen planus [65], actinic prurigo [66] ICAM-1 is expressed
on keratinocytes and/or Langerhans cells. ICAM-1 is expressed on melanocytes at
the margin of active vitiligo lesions [67], while it is expressed on
fibroblasts in cases of scleroderma [68]. ICAM-1 expression on
keratinocytes and melanocytes and its upregulation on endothelial cells are
induced by several inflammatory cytokines such as IFN-γ, TNFa, IL-1, IL-6 and IL-7 [5, 47, 63, 69]. The expression of ICAM-1
is usually associated with the presence of an activated inflammatory infiltrate
nearby, which contributes to the production of inflammatory mediators and
probably to the pathologic changes that result.
Allergic contact dermatitis (ACD)
Beside the role played by LFA-1 in the afferent
or sensitization phase of ACD mentioned before, a further role of integrins is
evident during the efferent or effector limb. Antigen specific memory T-cells
and other inflammatory cells invade the skin to cause the response clinically
recognized as ACD [70]. Skin-homing memory Th1-cells express LFA-1, a4b1 and CLA, which interact with their corresponding ligands ICAM-1,
VCAM-1 and E-selectin, expressed on endothelial cells until they migrate out of
the blood vessels. Inflammatory cytokines and chemokines regulate the process,
and among a number of effects, induce ICAM-1 on epidermal cells [59, 60].
LFA-1+ T-cells then head toward the ICAM-1+ epidermal
cells. They secrete IL-2, and IFN-gamma and other cytokines, which activate
cytotoxic T-cells, natural killer cells, macrophages and mast cells. The
collection of all these cells with their mediators is responsible for epidermal
spongiosis and dermal infiltrate, which are the histologic marks of ACD [70].
Psoriasis
Psoriasis is unique because it represents
excessive but controlled cellular
proliferation and inflammation. The exact pathogenetic mechanisms remain
unclear, however it is now regarded as T-cell dependent disease. Th1 cells are
recruited in a manner comparable to that of ACD. Psoriatic keratinocytes express
ICAM-1 and MHC-II in response to cytokines produced by T-cell [64].
There is increased expression of a3, a5
and a6
b1 integrins in suprabasal position [71]. Suprabasal integrin
expression is associated with epidermal hyperproliferation and could contribute
to the onset of psoriasis [72, 73, 74].
Leukocyte Adhesion deficiency (LAD)
Two types of leukocyte adhesion deficiency (LAD)
have been recognized, which share common clinical manifestations. The first,
LAD1, is a rare autosomal recessive disorder. It results from deficiency of
b2 integrin, leading to absence or deficiency of LFA-1, Mac-1 and p150,95
from the surface of neutrophils, monocytes and lymphocytes, which show impaired
chemotaxis and phagocytosis [75]. LAD2 results from genetic defect in
fucose synthesis, which is a constituent of the ligands for P- and E-selectin [5].
Patients with LAD1 have frequent skin infections,
mucositis, and otitis. The skin infections often present as necrotic abscesses
that resemble pyoderma gangrenosum, but the inflammatory response and production
of purulent material are impaired. Patients may have delayed separation of the
umbilical cord. Cellulitis of the face and perirectal area is common. Gingivitis
with periodontitis results in loss of teeth. Life-threatening severe bacterial
or fungal infections may occur. Poor wound healing leads to paper-thin or
dysplastic cutaneous scars [75].
The severity of clinical involvement is
proportional to the degree of glycoprotein deficiency. Patients with complete
deficiency (0-2% expression) die by 2 years of age of overwhelming sepsis, while
those with partial deficiency (10-20% expression) usually survive into adulthood[5]. Bone marrow transplantation represents the current treatment
option. The normal CD18 subunit gene has been introduced into hematopoietic stem
cells and may provide the basis for future gene therapy [75].
Integrins and Cutaneous Malignancy
Integrins may promote tumor growth directly
through their increased expression on the surface of tumor cells. These
integrins facilitate cell growth by induction of proliferative signaling
pathways and/or by facilitating the invasion of their surroundings. Indirectly,
increased integrin expression on blood vessels associated with tumors, may
enhance their growth by facilitating angiogenesis. Since tumorigenesis is a
multi-step process, the timing of altered integrin expression may be critical,
and early changes may have a greater effect on the course of the disease than
the pattern of integrin expression that characterizes the mature tumor [23].
Thus all malignancies, including those related to the skin bear variable forms
of integrin expression. Discussed below are squamous cell carcinoma and
malignant melanoma because of their relevant importance to the dermatologist.
Squamous cell
carcinoma
Squamous cell carcinoma reveals considerable
variation in integrin expression within the same tumor and between different
tumors. Normal expression, overexpression and focal or extensive loss of
expression of the major keratinocyte integrins have all been observed, together
with de novo expression of other integrins including
avb6[23].
Normally,
a6b4 integrin disappears as keratinocytes differentiate and move upwards in
the epidermis. Increased
a6b4
integrin expression correlates with a high risk of tumor progression in
stratified squamous epithelia [76]. Alpha 6 beta 4 overexpression in
suprabasal keratinocytes has been associated with poor prognosis in human oral
cancer [77]. Within a given tumor, both overexpression of
a6b4 in the suprabasal layers and focal loss at the tumor margin could be
observed [76, 78].
On the other hand, expression of
a6b4
is maintained in many invasive carcinomas in the absence of hemidesmosomes where
it is associated with the actin cytoskeleton and promotes migratory capability
of carcinoma cells [79].
Malignant
Melanoma (MM)
Increased expression of
avb3 was reported in malignant melanoma (MM), undifferentiated
neuroblastoma, highly metastatic breast carcinoma and prostatic adenocarcinoma
cell lines, which suggests its role in promoting rapid growth and metastasis of
aggressive tumors [5]. This role is supported by two basic biological
observations: avb3
ligates provisional matrix proteins such as fibronectin, fibrin, vitronectin and
thrombospondin, that are deposited in the inflammatory sites around tumors; and
avb3 binds and activates certain cell-surface-associated MMPs, which
facilitate degradation of ECM and tumor progression [80]. In MM as in
other aggressive tumors, the need for a good vascular supply is mandatory for
their growth and metastasis. Increased expression of the angiogenesis marker,
avb3, on endothelial cells supplying MM has been implicated in tumor growth
and sustenance [81]. Other than
avb3, integrins of the
b1 family expressed on MM cell lines have been implicated in tumor
metastasis [82, 83, 84].
Integrins
as Potential Drug Targets
The elucidation of the role of inflammatory
cells, their soluble mediators, adhesion molecules and signal transduction
pathways in the pathogenesis of diseases, helped in the development of new
targeted therapies, also known as biologics. T-cell mediated
diseases have received particular attention. As regards dermatological diseases,
extensive research has focused on psoriasis, a T-cell mediated disease [64].
Among the new therapeutic modalities, efaluzimab
is directed against CD11a or the
a-integrin
subunit of LFA-1 [85]. Efaluzimab (Raptiva) is a humanized monoclonal
antibody indicated for moderate to severe psoriasis. During efaluzimab
treatment, T-cell CD11a was downregulated, together with histological evidence
of epidermal thinning, decreased number of epidermal and dermal CD3+ T-cells,
decreased T-cell CD11a availability, and decreased keratin 16 and ICAM-1
expression [85].
References
1. Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549-54.
2. Berman AE, Kozlovz NI, Morozevich GE. Integrins: Structure and Signaling. Biochemistry (Moscow); 2003:1284-99.
3. Xiong JP, Stehle T, Goodman SL, Arnaout MA. New insights into the structural basis of integrin activation. Blood 2003; 102: 1155-9.
4. Parish WE. Inflammation. In: Rook, Wilkinson, Ebling Textbook of Dermatology. RH Champion, JL Burton, DA Burner, SM Breathnach (eds). Sixth edition, Blackwell Science Ltd. Oxford, London, Edinburgh, Massachusetts, 1998, p 229-276.
5. Clark RA, Tonnesen MG. Integrins in skin biology and pathophysiology. In Biology of the Skin. RK Freinkel, DT Woodley (eds). 1st edition. Parthenon Publishin Group Inc. New York. 2001 p333-352.
6. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-87.");
7. Green LJ, Mould AP, Humphries. The integrin beta subunit. Int J Biochem Cell Biol. 1998;30:179-84.
8. Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311-39.
9. Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha v beta 3. Science 2001; 294:339-345.
10. Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand Binding to Integrins. J. Biol. Chem. 2000; 275, 21785-88.
11. Hynes RO: Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69:11-25.
12. Ginsberg MH, Du X, Plow EF. Inside-out integrin signaling. Curr Opin Cell Biol. 1992; 4: 766-71.
13. Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000 ;113:3563-71.
14. Marcantonio EE, Guan JL, Trevithick JE, Hynes RO. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990;1:597-604.
15. O'Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047-59.
16. Howe AK, Aplin AE, Juliano RL. Anchorage-dependent ERK signaling--mechanisms and consequences. Curr Opin Genet Dev. 2002 Feb;12(1):30-5.
17. Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001; 11:48-53.
18. Yancey KB, Allen DM. The Biology of the Basement Membrane Zone. In Dermatology. JL Bolognia, Jorizzo JL, Rapini RP (eds). First edition. Mosby. London, Edinburg, New York, Philadelphia, St Lois, Sydney, Toronto 2003 p435-48.
19. Levy L, Broad S, Diekman D, Evans RD, Watt FM. Beta1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol Biol Cell. 2000; 11: 453-66.
20. Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729-38.
21. Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002; 21: 3919b-26.
22. Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J. Cell Biol. 2000; 149, 969-982.
23. Hopkinson SB, Baker SE, Jones JC. Molecular genetic studies of a human epidermal autoantigen (the 180 kD bullous pemphigoid antigen/ BP180): identification of functionally important sequences within the BP 180 moleculae and evidence for an interaction between BP 180 and alpha 6 integrin. J Cell Biol. 1995; 130:117-25.
24. Marchisio PC, Bondanza S, Cremona O, Cancedda R, De Luca M. Polarized expression of integrin receptors (a6b4, a2b1, a3b1 and avb5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J Cell Biol. 1991; 112; 761-73.
25. Borradori L, and Sonnenberg A. Structure and function of hemidesmosomes: More than simple adhesion complexes. J Invest Dermatol. 1999; 112:411-8.
26. Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001; 13:541-5.
27. Rabinovitz I, Gipson IK, Mercurio AM. Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion. Mol Biol Cell. 2001;12:4030-43.
28. Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949-60.
29. Santoro MM, Gaudino G, Marchisio PC. The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell. 2003;5:257-71.
30. Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999; 112: 2615-29.
31. Shimizu H, Suzumori K, Hatta N, Nishikawa T. Absence of detectable alpha 6 integrin in pyloric atresia-junctional epidermolysis bullosa syndrome. Application for prenatal diagnosis in a family at risk for recurrence. Arch Dermatol. 1996;132:919-25.";
32. Niessen CM, van der Raaij-Helmer MH, Hulsman EH, van der Neut R, Jonkman MF, Sonnenberg A. Deficiency of the integrin beta 4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. J Cell Sci.1996;109:1695-706.
33. Ashton GH, Sorelli P, Mellerio JE, Keane FM, Eady RA, Mc Garth JA. Alpha 6 beta 4 integrin abnormalities in junctional epidermolysis bullosa with pyloric atresia. Br J Dermatol. 2001; 144:408-14.
34. Takizawa Y, Shimizu H, Nishikawa T, Hatta N, Pulkkinen L, Uitto J. Novel ITGB4 mutations in a patient with junctional epidermolysis bullosa-pyloric atresia syndrome and altered basement membrane zone immunofluorescence for the alpha6beta4 integrin. J Invest Dermatol. 1997; 108:943-6.
35. Murakami H, Nishioka S, Setterfield J, Bhogal BS, Black MM, Zillikens D, Yancey KB, Balding SD, Giudice GJ, Diaz LA, Nishikawa T, Kiyokawa C, Hashimoto T. Analysis of antigens targeted by circulating IgG and IgA autoantibodies in 50 patients with cicatricial pemphigoid. J Dermatol Sci. 1998; 17:39-44.
36. Di Persio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg PA, Hynes RO. Alpha 3 beta 1 integrin is require for normal development of the epidermal basement membrane. J Cell Biol. 1997; 137: 29-4.
37. Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quarantana V. Induction of cell migration by matrix metalloproteinase-2 cleavage of laminin 5- Science 1997; 277:225-8.
38. Chen JD, Kim JP, Zhang K, Sarret Y, Wynn KC, Kramer RH, Woodley DT. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993;209:216-23.
39. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. Collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997; 137:1445-57.
40. Clark RAF. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol 1990; 94 (Suppl): 128-34S.
41. Gailit J, Welch MP, Clark RAF. TGF beta1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994; 103: 221-7.
42. Huhtala P, Humphries MJ, Mc Carthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins reulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995; 129: 867-79.
43. Clark RAF, Ashcroft GS, Spencer M-J, Larjava H, Ferguson MWJ. Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br J Dermatol. 1996; 135 : 46-51.
44. Clark RAF. Wound Healing. In Fitzpatrick’s Dermatology in General Medicine. 5th edition. IM Freedberg, AZ Eisen, K Wolff, KF Austen, LA Goldsmith SIK Katz, TB Fitzpatrick (eds) Mc Graw Hill Companies Inc. 1999 Chapter 27. e-Edition.
45. Ruggeri ZM. Von Willebrand factor and fibrinogen. Curr Opi Cell Biol 1993; 5:898-906.
46. Du X, Ginsberg MH. Integrin alpha IIb beta 3 and platelet function. Thromb Haemost 1997; 78: 96-100.
47. Fabbri M, Bianchi E, Fumagalli L, Pardi R. Regulation of lymphocyte traffic by adhesion molecules. Inflamm Res 1999; 48: 239-46.
48. Shaw RJ, Doherty DE, Ritter AG, Benedict SH, Clark RA Adherence-dependent increase in human monocyte PDGF(B) mRNA is associated with increases in c-fos, c-jun, and EGF2 mRNA. J Cell Biol 1990; 111:2139-48.
49. Clark RAF, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982; 79:264-9.
50. Adams JC, Watt FM: Expression of beta1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991; 115:829-41.
51. Kubo M, Van de Water L, Plantefaber LC, Mosesson MW, Simon M, Tonnesen MG, Taichman L, Clark RA. Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J Invest Dermatol. 2001;117:1369-81.
52. Greiling D, Clark RAF: Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci 1997; 110:861-70.
53. Gailit J, Xu J, Bueller H, Clark RA. Platelet-derived growth factor and inflammatory cytokines have differential effects on the expression of integrins alpha1 beta1 and alph5 beta1 by human dermal fibroblasts in vitro. J Cell Physiol 1996; 169:281-9.
54. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003; 200: 500-3.
55. Grinnell F: Fibroblasts, myofibroblasts, and wound contraction.J Cell Biol 1994; 124:401-4.
56. Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000; 5:40-6.
57. Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 1993; 121: 163-70.
58. Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol 1995; 57: 827-72.
59. Luscinskas FW Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA Jr. Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol.1994; 125:1417-27.
60. Luscinskas FW et al: P-selectin and VCAM-1 mediate rolling and arrest, respectively, of CD4 + T lymphocytes on TNF-alpha-activated vascular endothelium under flow. J Exp Med. 1995; 181:1179-86.
61. Detmar M. Vascular Biology. In Dermatology. JL Bolognia, Jorizzo JL, Rapini RP (eds). First edition. Mosby. London, Edinburg, New York, Philadelphia, St Lois, Sydney, Toronto 2003 p 1987-98.
62. Lub M, van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today. 1995; 16; 479-83.
63. Norris D. Cytokine modulation of adhesion molecules in the regulation of immunologic cytotoxicity of epidermal targets. J Invest Dermatol 1990; 95: 111S-20S.
64. Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002; 46:1-23.
65. Villarroel Dorrego M, Correnti M, Delgado R, Tapia FJ. Oral lichen planus: immunohistology of mucosal lesions. J Oral Pathol Med. 2002;31:410-4.
66. Umana A, Gomez A, Duran MM, Porras L. Lymphocyte subtypes and adhesion molecules in actinic prurigo: observations with cyclosporin A. Int J Dermatol. 2002 Mar;41(3):139-45.
67. Al-Badri AMT, Foulis AK, Todd PM, Garioch JJ, Gudgeon JE, Stewart DG, Gracie JA, Goudie RB. Abnormal expression of MHC class II and ICAM-1 by melanocytes in vitiligo. J Pathol 1993; 169: 203-06.
68. Sollberg S, Krieg T. New aspects in scleroderma research. In Arch Allergy Immunol. 1996; 11:330-6.
69. Kirnbauer R, Charvat B, Schauer E, Kock A, Urbanski A, Forster E, Neuner P, Assmann I, Luger TA, Schwarz T. Modulation of intercellular adhesion molecule-1 expression on human melanocytes and melanoma cells: Evidence of a regulatory role of IL-6, IL-7, TNF-b and UVB light. J Jnvest Dermatol 1992; 98: 320-26.
70. Belisto DV. Allergic Contact Dermatitis. In Fitzpatrick’s Dermatology in General Medicine. 5 edition. IM Freedberg, AZ Eisen, K Wolff, KF Austen, LA Goldsmith SIK Katz, TB Fitzpatrick (eds) Mc Graw Hill Companies Inc. 1999 Chapter 27. e-Edition.
71. Kellner I, Konter U, Sterry W. Overexpression of extracellular matrix receptors (VLA-3, 5 and 6) on psoriatic keratinocytes. Br J Dermatol. 1991;125:211-6.
72. Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in development al defects and a phenotype resembling psoriasis. Cell. 1995; 83: 957-68.
73. Romero MR, Carroll JM, Watt FM. Analysis of cultured keratinocytes from a transgenic mouse model of psoriasis: effects of suprabasal integri expression on keratinocyte adhesion, proliferation and terminal differentiation. Exp Dermatol. 1999; 8: 53-67.
74. Haase I, Hobbs RM, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001; 108: 527-36.
75. Paller AS, Nanda V, Spates C, O'Gorman M. Leukocyte adhesion deficiency: Recurrent childhood skin infections. J Am Acad Dermatol 1994. 31:316-9.
76. Owens DM, Romero MR, Gardner C, Watt FM. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J Cell Sci. 2003;116:3783-91.
77. van Waes C, Kozarsky KF, Warren AB, Kidd L, Paugh D, Liebert M, Carey TE. The A9 antigen associated with aggressive human squamous cell carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res. 1991; 51: 2395-402.
78. Downer CS, Watt FM, Speight PM. Loss of alpha 6 beta 4 integrin subunits coincides with loss of basement membrane components in oral squamous cell carcinomas. J Pathol. 1993; 171: 183-90.
79. Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion-lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001;11:129-41.
80. Gladson CL, Hancock S, Arnold MM, Faye-Petersen OM, Castle berry RP, Kelly DR. Stage-specific expression of integrin alpha v beta3 in neuroblastic tumors. Am J Pathol. 1996; 148:1423-34.
81. Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumorangiogenesis in vivo by alpha v beta 3-targeted magnetic resonance imaging. Nature Med. 1998; 408-14.
82. Knutson JR, Iida J, Fields GB, Mc Carthy JB. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell. 1996; 7:383-96.
83. Melchiori A, Mortarini R, Carlone S, Marchisio PC, Anichini A, Noonan DM, Albini A. The alpha 3 beta 1 integrin is involved in melanoma cell migration and invasion. Exp Cell Res. 1995; 219: 233-42.
84. Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha 6 beta 1 integrin by laminin G peptides. J Biol Chem 1996; 271: 27221-4.
85. Leonardi CL. Efaluzimab: An overview. J Am Acad Dermatol. 2003;49 (Suppl):98-104S.
© 2005 Egyptian Dermatology Online Journal
|
