|
|
Abstract
Cutaneous metastases can be the presenting sign of internal malignancy, the first evidence of disseminated neoplastic disease or metastases developing late in the course of the disease, which represent a poor prognostic sign. Furthermore, they may serve as
an easy to follow marker during the specific anti-cancer treatment. Any unusual skin lesion should be histopathologically examined, since it may be a guide to prompt diagnosis and treatment of an underlying malignancy. Introduction
Cutaneous metastases may occur as the initial manifestation of internal malignancy or late in the course of the disease. Furthermore, they can be the first sign of disseminated neoplastic disease or an important presenting feature of recurrence after successful therapy. The course of cutaneous metastases may also be used as a reflection of the behavior of the internal lesions, in particular their response to systemic chemotherapy. Presence of cutaneous metastases may progress the stage of an internal malignancy and hence prognosis. Tumor cells metastasize the skin through several routes, namely direct invasion from underlying structures, extension through lymphatics, embolization through lymphatics and blood vessels, and accidental implantation at surgery. In general, cutaneous metastases are associated with advanced systemic cancer disease, and expected survival is less than one year [1,2]. Cutaneous metastases are the first sign of extranodal disease in 7.6% and in general occur in 0.7% to 9.0% of all cancer patients [3,4]. Incidence of various tumors metastasizing to the skin correlates with the sex-wise frequency of occurrence of various primary malignancies [3]. Breast carcinoma (69%) is the commonest cause of cutaneous metastases in women followed by carcinoma of large intestine (9%), lungs and ovaries (4%). Among men, the primary sites of carcinoma with cutaneous metastases in decreasing order are the lungs (24%), large intestine (19%), oral cavity (12%), kidney and stomach (6% each). In addition, Kaposi's sarcoma, lymphoma, and leukemia can cause cutaneous lesions [5]. The following cases are reported to highlight the importance of cutaneous metastases as a presenting sign of internal malignancy (case 1), as the first evidence of disseminated neoplastic disease (cases 2, 3), and as metastases developing late in the course of the disease (case 4). Case reports
Case 1 A 58-year-old woman gradually developed multiple asymptomatic erythematous and edematous papules over the pubic area of two months duration (fig. 1a). Six months before, she had developed an asymptomatic left inguinal lymph node enlargement in addition to night fever and sweating for which she received oral antibiotics without improvement. Histopathological examination of skin lesions showed the presence of an atypical mononuclear cell infiltrate extending into the subcutaneous fat (fig 1c). The infiltrate was composed of large cells
with vesicular nuclei and prominent nucleoli as well as small lymphocyte-like cells (fig 1d). The findings were consistent with anaplastic large cell lymphoma (ALCL). Immunostaining was positive for CD3, CD4, CD30, epithelial membrane antigen (EMA) (fig. 1f) and negative for CD15 and cutaneous lymphocyte-associated antigen (CLA). Lymph node biopsy showed effacement of nodal architecture by diffuse infiltrate of large malignant lymphoid cells with multinucleated giant cells (fig. 1e). Foci of necrosis and microabscesses could be seen. Immunostaining for CD3, CD4, CD30 (Ki-1) was strongly positive. An abdomino-pelvic computerized tomography (CT scan) revealed the presence of hepatosplenomegaly together with enlarged paraaortic and pelvic lymph nodes, especially at the inguinal, obturator and iliac regions. No other evidence of systemic involvement could be detected. The patient was staged as VIa T1 N3 M0. Accordingly, the diagnosis of systemic form of Non-Hodgkin CD30+ve ALCL was made and treated with CHOP-MTX (cyclophosphamide, doxorubicin, prednisone, vincristine and methotrexate). Three weeks later, the skin lesions almost disappeared (fig. 1b) and abdomino-pelvic CT scan showed marked decrease in the paraaortic and pelvic lymph nodes.
There was No evidence of recurrence after 6 months of follow up.
 | Fig 1:
Skin metastases from CD30-positive ALCL (a) before and
(b) after
CHOP-MTX: (c) Atypical mononuclear cell infiltrate extending into
the subcutaneous fat (HE, x100); (d) Infiltrate composed of large
cells with vesicular nuclei with prominent nucleoli as well as small
lymphocyte-like cells (HE, x400); (e) Lymph node biopsy showing
diffuse infiltrate of large malignant lymphoid cells with
multinucleated giant cells (HE, x100). (f) Positivity for CD3; |
|
Case 2 A 22 year old female presented with a tender erythematous nodule (6 cm in diameter) over the right vertex area of the scalp over 4 months (fig. 2a). The lesion showed central ulceration with purulent discharge. No lymphadenopathy or organomegaly could be clinically detected. Two years before, the patient was treated for primary cerebral lymphoma by open craniotomy and resection followed by 25 sessions of radiotherapy. Histopathological examination showed findings similar to those of case 1, which were consistent with ALCL. Immunohistochemical staining for CD3, CD30 (Ki-1) was strongly positive. Axial contrast CT scan of the brain showed a hyperdense mass in left cerebral hemisphere without bony invasion. Screening for bone metastases revealed the presence of sclerotic metastases of L2 vertebra (fig 2b). According to these findings, the patient was diagnosed as having systemic form of CD30+ ALCL with secondary skin involvement (stage VIb T3 N0 M1). The patient received systemic chemotherapy but after initial decrease in the size of the lesions, gradual increase in number and size occurred and the patient died a few weeks later.
 | Fig
2: (a) Scalp metastases from CD30-positive anaplastic large cell
lymphoma. Histopathology was identical to case 1; (b) Axial CT
showing the primary lesion in the left cerebral hemisphere and
lateral radiograph sclerotic metastases of L2 vertebra. |
|
Case 3 A 62-year-old woman presented with multiple asymptomatic erythematous glistening papules on the front of the chest and abdomen and extending to the shoulders, buttocks and upper thighs of 3 months duration (fig. 3a). Four years before, the patient had been diagnosed as having invasive duct carcinoma of the right breast for which she had a modified radical mastectomy followed by radiotherapy. One year later, the patient was diagnosed as having invasive duct carcinoma of the left breast, which was also treated by modified radical mastectomy followed by radiotherapy. Histopathological examination of a skin papule showed invasion of the epidermis and dermis by malignant cells within a mucinous stroma indicating the presence of metastatic mucinous carcinoma (fig. 3b). Bone scan showed metastatic foci at the left side of the body of L4 and the posterior part of the left iliac crest (stage VI).
 | Fig
3: (a) Metastatic mucinous breast carcinoma;
(b) Malignant cells
within mucinous stroma invading the dermis (HE, x100). |
|
Case 4 A 44-year-old male patient presented with multiple firm and tender nodules measuring 2-3 cm in diameter; some of
which showed central black crust of 20 days duration (fig. 4a). Twelve months before, he had been diagnosed as a case of acute myeloid leukemia (AML) (M1-M2). At that time WBC count was 25000/mm3, Hb 11g/dl (13-16 g/dl) and platelets 170000/mm3 (normal
range 200,000-400,000/mm3). Erythrocyte sedimentation rate was 130 mm/h and lactate dehydrogenase was 1225 IU/L (normal range 100-190 IU/L). A bone marrow biopsy showed hypercellularity, with myeloid and erythroid series showing dysplastic features, lymphocytes: 60% of absolute neutrophilic count (ANC), 20% blast cells, Plasma cells: 1% of ANC. Megakaryocytes were abundant. Myeloperoxidase (MP)-positive flowcytometry. CD33, CD13, CD34, CD15, and HLA DR were positive. Pelviabdominal ultrasonography showed hepatomegaly, bilateral renal stones, left hydroureter and hydronephrosis. The patient was treated with doxorubicin, cytarabine and etoposide every three months. A skin biopsy showed little epidermal involvement with an underlying Grenz zone. A deep dermal monocytic infiltrate reaching the subcutaneous tissue was present. The infiltrate was accentuated perivascularly and periadnexally. The cells were large with an oval, vesicular nucleus and hyperchromatic basophilic cytoplasm (fig. 4b). Collagen fibers separated by leukemic cells (Indian filing) were evident in some areas (fig. 4c). Immunostaining for CD34, CD68 was positive confirming the diagnosis of leukemia cutis. On presentation of skin lesions the general condition of the patient worsened with a CBC showing Hb: 6.9 gm/dl, WBCs: 21000/mm3, PTL: 22000/mm3, RBCs: 173 mg/dl, LDH: 800. The patient died within two months of appearance of skin lesions.
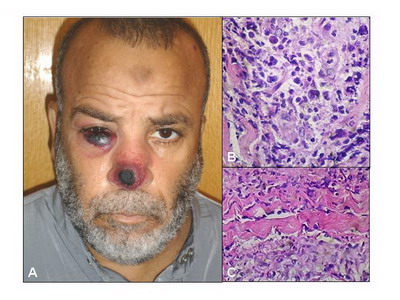 | Fig
4: (a) Leukemia cutis in a patient with AML;
(b) Pleomorphic
monocytic cells with an oval, vesicular nucleus and basophilic
cytoplasm (HE, x400); (c) Collagen fibers separated by leukemic
cells (Indian filing) (HE, x400). |
|
Discussion
The occurrence of cutaneous spread of internal malignancy is rather rare, however the incidence of malignancies is rapidly increasing and it is likely that skin metastases will be more frequently encountered
[6]. Such metastases offer an easily accessible tissue sample for rapid histopathological diagnosis of the malignancy and response of cutaneous metastatic lesions to chemotherapy mirrors the response of the primary tumor. A thorough clinical examination of the skin for any metastasis is therefore mandatory for a patient with any type of cancer. The triad of unexplained fever, inguinal lymphadenopathy, and skin lesions is highly suspicious for lymphoma as shown in case one in this report. Histopathological and immunohistochemical examination of skin and lymph node biopsies were consistent with the diagnosis of CD30 positive ALCL of the skin together with malignant regional lymph node involvement. Although the primary cutaneous form of ALCL is defined by skin-only involvement without systemic dissemination at presentation, draining regional lymph node involvement occurs in approximately 25% of patients with only skin lesions. Whether the patients only showing draining regional lymph node involvement, as in our case, should be considered to have a primary cutaneous or systemic form remains controversial
[7]. Primary cutaneous cases are reported to lack EMA and Anaplastic Lymphoma Kinase (ALK) but express CLA
[8], whereas ALCL that present with systemic extracutaneous disease (with or without skin involvement) are CLA- and EMA/ALK+ in 60% of cases
[9]. The differentiation of primary cutaneous and systemic disease is of great prognostic value. The 5-year survival rate of the former is 90% with occasional spontaneous regression (up to 25% of cases) of the skin lesions. The prognosis of the systemic form with secondary cutaneous involvement depends on the expression of the ALK protein. Patients with expression of ALK have a 5-year survival rate ranging from 70-80%, while survival ranges from 15-30% without ALK expression (10). ALK positive cells are usually also positive for EMA
[11]. Our case was CLA - but EMA+ indicating that it is a systemic form of ALCL with a good prognosis. The skin lesions were the first manifestation of the underlying malignancy and their resolution with treatment was a valuable indicator of the response of the primary malignancy to therapy. A solitary cutaneous nodule could be the first evidence of a metastatic disease in a known patient with systemic ALCL previously treated successfully as shown in the second case. Indeed, it is a good example of metachronous metastases, i.e., metastases developing years after the diagnosis and treatment of the primary malignancy. Because breast cancer is so common, cutaneous metastases are the most frequently encountered type in women in most clinical practices. Cutaneous involvement from breast carcinoma preferentially occurs in the skin overlying or proximal to the area of the primary tumor by direct extension or through lymphatic vessels. Nodular carcinoma, inflammatory or erysipeloides carcinoma, telangiectatic and "en cuirasse" carcinoma are the typical clinical manifestations of the lymphatic dissemination to the skin
[5]. The patient in this report had cutaneous metastatic mucinous carcinoma clinically evident as an erythematous glistening (due to mucinous content) papular eruption, which was found to be a part of systemic metastatic disease. Mucinous carcinoma accounts for 3% of breast cancers and is more common among older women. This rare type of invasive breast cancer is formed by mucin-producing cancer cells and carries a better prognosis than the more common types of invasive breast cancer. Colloid carcinoma is another name for this type of breast cancer
[12]. Acute myelogenous leukemia (AML) shows the second highest rates of leukemia cutis after adult T-cell leukemia/lymphoma. In general, leukemia cutis is a poor prognostic sign. It has been shown that in the presence of leukemia cutis in AML or chronic myeloid leukemia, the disease course is aggressive and the length of survival is short (7.5 and 9.4 months respectively)
[13]. Baer et al. showed that 90% of patients with AML and leukemia cutis had other sites of extramedullary involvement. In case 4 the appearance of skin lesions in a patient with AML was associated with a rapid decline in blood indices and death within 2 months despite the initial improvement
[14]. References
1. Brownstein MH and Helwig EB: Patterns of cutaneous metastasis. Arch Dermatol 105: 862-868, 1972.
2. Arun J and Prasad SS: Cutaneous metastatic adenocarcinoma. Indian J Dermatol, Venereol Leprol 67: 207-208, 2001.
3. Spencer PS and Helm TN. Skin metastases in cancer patients. Cutis 39:119-121, 1987.
4. Johnson WC: Metastatic carcinoma of the skin: incidence and dissemination. In Lever's Histopathology of the Skin, 8th edn, Edited by Elder D, Elenitsas R, Jaworsky C, Johnson Jr B, Lippincott-Raven, Philadelphia, 1011-1018; 1997.
5. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol 33: 161-185, 1995.
6. Beljaards RC, Kaudewitz P, Berti E, Gianotti R, Neumann C, Rosso R, Paulli M, Meijer CJLM, Willemze R: Primary cutaneous CD30-positive large cell lymphoma: Definition of a new type of cutaneous lymphoma with a favorable prognosis. A European multicenter study on 47 cases. Cancer 71:2097-2104, 1993.
7. Wollina U, Graefe T, Konrad H, Sch?nlebe J, Koch A, Hansel G, Haroske G, K?stler E. Cutaneous metastases of internal cancer. Acta Dermatoven APA, 13:79-84, 2004
8. de Bruin PC, Beljaards RC, van Heerde P, van der Valk P, Noorduyn LA, Van Krieken JH, et al. Differences in clinical behaviour and immunophenotype between primary cutaneous and primary nodal anaplastic large cell lymphoma of T-cell or null cell phenotype. Histopathology 23: 127-135; 1993
9. Pileri SA, Piccaluga A, Poggi S, Sabattini E, Piccaluga PP, de Vivo A, Falini B, Stein H. Anaplastic large cell lymphoma: update of findings. Leuk Lymphoma 18: 17-25; 1995.
10. Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, Verhoef G, Menestrina F, Todeschini G, Paulli M, Lazzarino M, Giardini R, Aiello A, Foss HD, Araujo I, Fizzotti M, Pelicci PG, Flenghi L, Martelli MF, Santucci A.l. ALK+ lymphoma: clinico-pathological findings and outcome. Blood 93: 2697-2706; 1999.
11. Ten Berge R L, Oudejans J J, Dukers D F, and Meijer C J L M. Anaplastic large cell lymphoma: what's in a name? J Clin Pathol 54: 494 - 495; 2001.
12. Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Annals of Surgical Oncology, 447-451, 1998.
13. Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol 40: 966-78; 1999.
14. Baer MR, Barcos M, Farrell H, Raza A, Preisler HD.: Acute myelogenous leukemia with leukemia cutis. Eighteen cases seen between 1969 and 1986. Cancer 63: 2192-200; 1989.
© 2007 Egyptian Dermatology Online Journal |




