|
| Abstract
Background
Pityriasis rosea (PR) is an acute, self-limiting papulosquamous skin disease. T-helper lymphocytes play an important role in the pathogenesis of PR. However, the role of cytotoxic T-lymphocytes is poorly understood.
Objective
To investigate the immune profile of mixed inflammatory cell infiltrate in skin lesions with PR.
Methods
The study included 10 biopsy specimens from lesional skin with clinical
diagnosis of PR . Ten biopsy specimens from normal skin served as a control.
Monoclonal antibodies were used for targeting antigenic epitopes on several
cell types including: CD3 (for T-cells), CD20 (for B-cells), CD68 (for macrophage/dendritic cells), and Granzyme B (Gr B) (for active cytotoxic T-cells).
Results
Levels of CD3 +ve, CD68 and Gr B were significantly higher in lesional skin as compared to normal skin (p<0.05). CD3 +ve cells was the predominant cell population in lesional skin.
Conclusion
The finding of significantly increased numbers of CD3 +ve cells in skin lesions with PR indicates an important role of cell mediated immunity in the pathogenesis of the disease. A significantly increased number of Gr B +ve cells in all lesions suggested that a fraction of CD3 +ve cells have an active cytotoxic potential.
Introduction
Pityriasis rosea (PR) is an acute, self-limiting skin disease, affecting mainly children and young adults. It is characterized by a distinctive skin eruption and minimal constitutional symptoms. [1] The exact aetiology of PR is uncertain. However, epidemiological and clinical features of the disease suggest that an infective agent may be implicated. [2] Virus-like particles were detected ultrastructurally and herpes virus-like particles have been found in 71% of PR lesions. A previous study showed increased number of B-lymphocytes and, a decrease in T- lymphocytes in blood from patients with PR. [3] Histological changes of PR are not specific and may include acanthosis (irregular psoriasiform pattern), focal parakeratosis, spongiosis; and absence or decrease in granular cell layer. Isolated large epithelioid giant cells are infrequently encountered in the epidermis. [4] Electron microscopic studies have shown aggregations of tonofilaments, intracytoplasmic desmosomes and cytoplasmic vacuoles in the dyskeratotic cells of the epidermis. In addition, some electron-dense viral like particles were seen at time in the keratinocytes. [5] Peri-vascular lympho-histiocytic reaction is usually seen in the upper dermis in patients with PR. Immuno-histological studies demonstrated that majority of cells in upper dermal infiltrate consisted of helper/inducer T-cells. In addition, numerous Langerhans cells were seen in superficial peri-vascular infiltrate. [4] However, the role of mixed inflammatory cellular infiltrate in the pathogenesis of PR is poorly understood. The objectives of this study were to determine the site and extent of skin infiltrating mononuclear inflammatory cells in skin lesions with PR and to analyze the expression pattern of CD3 (T-cells), CD20 (B-cells), CD68 (macrophage/dendritic cells), and Granzyme B [GrB] (active cytotoxic T-cells) in these lesions as compared to normal skin.
Methods
This study was conducted between October, 2004 and January, 2006. Patients with clinical diagnosis of PR (n=10) were eligible for inclusion in the study. Biopsy specimens were obtained from lesional skin (4-6 mm punch). Ten specimens from normal skin served as control. The specimens were processed at Department of Pathology, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt. All immunohistochemical tests were performed at the Immunohistochemistry Laboratory, Assuit University Hospitals, Assuit, Egypt.
Pathological data Four-micron thick sections were stained with Hematoxyline and Eosin. Stained sections were examined for presence or absence of the following histological changes: 1) hyperkeratosis (compact, basketwave or lamellar); 2) parakeratosis; 3) hypogranulosis or hypergranulosis; and 4) acanthosis (regular, irregular and psoriasiform). Papillary and reticular dermis were examined for the presence or absence of fibroplasia and melanin incontinence. Skin adnexa were examined for the presence or absence of periadnexal inflammatory cell infiltrate. The hypodermis was evaluated for the presence of any pathological changes.
Inflammatory cell infiltrates Histological evaluation of inflammatory cell infiltrate (type and positioning) was done on histological sections (H&E stained sections) by counting cells in at least five different fields by two observers. The values were expressed as mean and standard error of mean (mean ± SEM).
Immunohistochemistry All immunohistochemical tests were performed as described by Hussien et al (2004) [6] . Formalin-fixed paraffin-embedded tissue sections were immunostained using peroxidase-labeled streptavidine biotin technique to detect reactivity for CD68 (histiocyte/dendritic cells), CD20 (B-lymphocytes), CD3 (T-lymphocytes), and Gr B (active cytotoxic T-cells). Primary monoclonal antibodies were used including PG-M1, GrB-7, L26, PC3/188A for CD68 , Gr B, CD20 and CD3 ; respectively (Dako Corporation, Carpinteria, USA). Working dilution of primary antibodies was prepared from reducing components (Dako Corporation, Catalogue # S3022, Carpinteria, USA). A universal staining kit LSAB2/HRP, Rabbit/mouse, Liquid DAB (Dako Corporation, Catalogue # K0673, Carpinteria, USA) was used according to the manufacturer instructions. Silane-coated slides were prepared to increase adherence of sections to glass surfaces. Formalin-fixed, paraffin-embedded sections were cut by microtome at 5-micron thickness, and mounted on the silane-coated glass slides. Sections were deparaffinized and rehydrated through graded alcohols to distilled water. Endogenous peroxidase activity was blocked with 0.6% hydrogen peroxide for ten minutes using peroxidase blocking reagent (Dako Corporation, Catalogue # K0673, Carpinteria, USA). The slides were washed in distilled water for three to five minutes.
Antigen retrieval [6] Fixation of tissue specimens in formalin leads to formation of excess aldehyde linkages which mask tissue antigens and thus prevent its localization by primary antibody. Antigen retrieval is therefore necessary to unmask antigen sites by breaking aldehyde bonds. Heat-induced antigen retrieval was used for immunostaining of CD68 , CD20 , and Gr B. Alternatively, enzyme-induced antigen retrieval was used for immunostaining of CD3 . Heat-induced antigen retrieval was done by immersing slides in 10mM sodium citrate buffer solution, pH 6.0. The latter was prepared by dissolving 10.5 gm of citric acid anhydrous in 200 ml distilled water. Slides were microwaved at a power of 650 watt (20 min for CD68 and 15 min for CD20 and Gr B). The amount of retrieval fluid in the container was regularly checked out and fresh buffer was regularly added to prevent slides from drying out. Then, containers were removed from the oven, and allowed to cool for 15 minutes at room temperature. Slides were washed several times in distilled water, and placed in phosphate buffer saline (PBS, pH 6.0) for five min. Enzyme induced antigen retrieval of CD3 was done by immersing slides in trypsin at a temperature of 37C º for three min. Slides were then removed from trypsin, washed for several times in distilled water, and two times in PBS for five minutes.
Staining steps [6] Excess PBS buffer was blotted off, and slides were dried except for tissue sections. The sections were incubated with normal goat serum to block nonspecific interactions. Sufficient amounts of primary monoclonal antibodies were added on tissue sections. The dilution differed according to the type of antibody. Anti-CD68 was provided in a diluted form whereas, CD20 , CD3 , and Gr B were used at dilutions of 1/200, 1/100, and 1/25; respectively. Slides were incubated with primary antibodies horizontally in a humid chamber at room temperature for 30 minutes. Excess reagent was thrown off, and slides were rinsed in PBS for five minutes for two consecutive times. The resulting immunocomplex was detected by a universal staining kit. After blotting off excess buffer, tissue sections were treated with biotinylated goat anti-polyvalent solution for 10-15 minutes at room temperature. The slides were rinsed in two consecutive jars of PBS for five min. The excess buffer was blotted off, and peroxidase-labelled streptavidin was applied for 10-15 minutes at room temperature. The slides were rinsed with PBS, and blotted. Then, slides were incubated with 14-diaminobenzidine and 0.06% H 2 O 2 for five minutes, washed in distilled water, and counter-stained using Mayer’s hematoxyline. The tissue sections were washed in tap water, dehydrated in alcohol, and cleared in xylene. Slides were air-dried, mounted in DPX then cover slipped.
Positive control In each staining run, tissue specific positive control slides were included. Lymph nodes with reactive lymphoid hyperplasia were used as a source for +ve control slides. CD68 , (histiocytes/dendritic cells) was obtained from lymph node sinuses; CD20 , (B lymphocytes) from mantle zone; CD3 , (T lymphocytes) from paracortex; and Gr B, (cytotoxic T lymphocytes) from paracortex [6] .
Negative control In each staining run, negative control slides were included to confirm validity of staining. For this purpose, additional tissue sections were stained in parallel, but with omission of the primary antibody as described by Hussien and Wood (2002) [7] .
Evaluation of immunostaining Corresponding histological sections were examined side by side with immunostained sections. In each case, the entire section was histologically examined by bright field microscope (Olympus, Olympus optical, Tokyo, Japan). Initial examination was done at low power magnification (X4 and X10) to detect sites of the antibody positivity, and then the high power magnification (X40) was used to evaluate the immunostaining. Positive staining was quantified by counting total cells in at least three different fields, and then positive cells were quantified (in epidermis and perivascular area). Mean value of these counts and standard error of mean were calculated. Reactivity for CD68 and Gr B was defined as granular brownish cytoplasmic staining. Reactivity for both CD20 and CD3 was defined as brownish membranous staining [6] . Results
The mean (± SEM ) age of patients with PR was (24 ± 1.4). Histopathological features (H & E) of normal and lesional skin
(Figure 1a & 1b; respectively) Epidermal changes Hyperkeratosis was present in all cases with predominance of lamellar pattern (9/10, 90%). Parakeratosis was present in 8/10 (80%) of cases. Acanthosis was found in all cases with irregular psoriasiform pattern. Kolliocytosis was found in 8/10 (80%) of cases. Dermal changes All cases showed variable inflammatory cell infiltrate in the papillary and reticular dermis. The pattern of this infiltrate was exclusively patchy in nature. The infiltrate was formed of variable admixture of lymphocytes and histiocytes. The number of lymphocytes was significantly higher in lesional skin from patients with PR as compared to normal skin (34.4 ± 1.6 Vs 4.2 ± 0.5; P < 0.05). The number of histiocytes was significantly higher in lesional skin from patients with PR as compared to normal skin (15.6 ± 1.3 Vs 4.6 ± 1.4; P < 0.05)
( table 1 ) . Table 1: Histological evaluation of inflammatory cell infiltrate in skin lesions with PR as compared to normal skin
| |
Normal skin (n=10) |
Lesional skin(n=10) |
P -Value (overall) |
|
Lymphocytes |
4.2 ± 0.5 |
.34.4± 1.6 |
< 0.05 |
|
Histiocytes |
4.6± 1.4 |
15.6±1.3 |
< 0.05 |
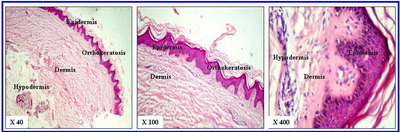 |
Figure 1a: Normal Skin (H & E) |
|
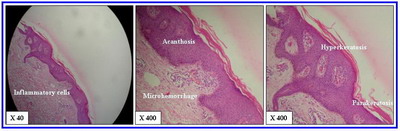 |
Figure 1b: Pityriasis Rosea (H & E) |
|
Immunohistochemical Features of normal and lesional skin
(Figure 2a & 2b; respectively) The percent of CD68 + cells was significantly higher in PR as compared to normal skin (14.9 ± 1.7 Vs. 4.0 ± 1.0; P < 0.05). The percent of CD3 + cells was significantly higher in PR as compared to normal skin (61.9 ± 5.4 Vs. 3.0 ± 1.1; P < 0.05). The percent of Gr B + cells was significantly higher in PR as compared to normal skin (5.0 ± 0.8 Vs. 0.6 ± 0.4; P < 0.05). CD20 + cells were absent in normal skin and present in lesional skin from PR (11.1 ± 2.4)
( table 2 ). Table 2: Immunohistochemical evaluation of skin lesions with PR as compared to normal skin
|
Skin biopsy |
Normal skin(n=10) |
Lesional skin(n =10) |
P- Value(overall ) |
|
Immune profile |
|
*CD68 |
4.0±1.0 |
14.9 ± 1.7 |
< 0.05 |
|
**CD20 |
0.0±0.0 |
11.1± 2.4 |
< 0.05 |
|
***CD3 |
3.0±1.1 |
61.9 ± 5.4 |
< 0.05 |
|
****Gr B |
0.6 ± 0.4 |
5 ± 0.8 |
< 0.05 |
* CD68 = macrophage/dendritic cells. ** CD20 = B-cells.
*** CD3 = T-cells.
**** Gr B = active cytotoxic T-cells.
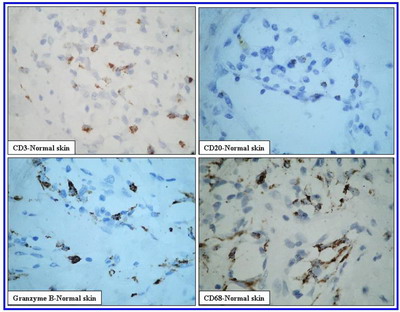
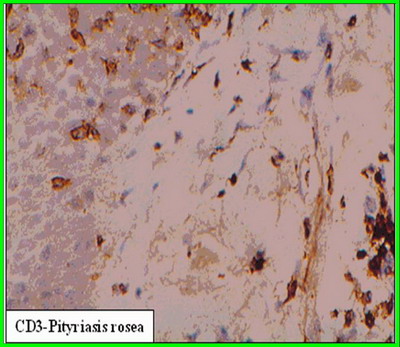
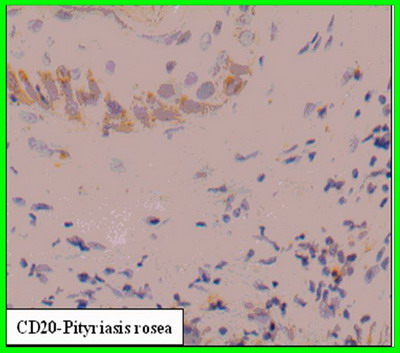
Figure 2a (X400): CD3, CD20, CD68 & Granzyme B expression
in normal skin. Reactivity for CD3 and CD20 appears as membranous
staining. Signals for CD68 and Granzyme B appear as diffuse
granular cytoplasmic staining. CD3 +ve and CD68 +ve cells are the
most predominant cell populations followed by Granzyme B +ve cells.
CD20 +ve cells are occasionally seen.
|
|
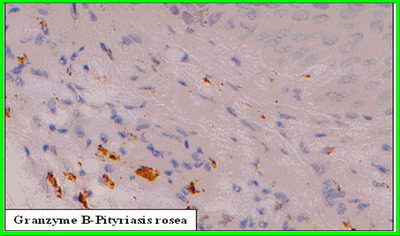 |
Figure 2b (X400): CD3, CD20, CD68 & Granzyme B expression
in lesional skin in PR. The reactivity for CD3 appears as brownish
membranous staining in T cells residing in papillary and reticular
dermis. The reactivity for CD20 appears as brownish membranous
staining in B cells residing in papillary and reticular dermis. The
reactivity for CD68 appears as diffuse granular cytoplasmic
staining in CD68 +ve cells residing in papillary and reticular
dermis. The reactivity for Granzyme B protein appears as diffuse
granular cytoplasmic staining in cytotoxic T cells residing in the
papillary and reticular dermis. |
|
Discussion
Despite the fact that PR is a common dermatological problem, its etio-pathogenesis is uncertain. Our results indicated an increased density of MICs in lesional skin from patients with PR as compared to normal control. Phenotypic characterization of the MICs in these lesions indicated that majority of cells were T-lymphocytes (CD3 +ve) and histiocytes (CD68 +ve). These findings agreed with a previous report of similar findings in psoriasis, atopic dermatitis, and other inflammatory dermatoses. [8] It has been suggested that these cells may participate in the immune responses resulting in the pathology associated with inflammation. The finding of high density of CD3 +ve cells in lesional skin from patient with PR, in our study, is in line with previous reports. [9,10] On the other hand, scarcity of CD20 +ve B lymphocytes in lesional skin, in our study, may suggest that humoral immunity does not play a significant role in the evolution of these lesions. However, the presence of B lymphocytes in a density higher than that of the adjacent normal skin suggests an intact but weak recruitment signals for these cells. This observation is consistent with that reported in a previous study. [11] Interestingly, some of T cells (CD3 +ve) had shown positive cytotoxic activity (Gr B +ve). Although present in small numbers, Gr B + cells counts in lesional skin of PR were significantly higher than normal controls. Previous studies suggested that cytotoxic T-cells may play an integral part in eliciting cutaneous inflammation in atopic dermatitis and allergic contact dermatitis. [12,13] However, this is the first report (to best of our knowledge) on GrB expression in skin lesions from patients with PR. Such finding may indicate a potential role for apoptotic cell death in the pathogenesis of these lesions. Analysis of MICs, in our study, in normal skin revealed the presence of lymphocytes and histiocytes as the predominant cell populations. Characterization of these immunocytes revealed that lymphoid cells were T cells (CD3 + ve), and no neutrophils, eosinophils, or plasma cells were detected. These findings agreed with earlier studies and support the view that the immune response in skin is essentially a cell mediated one. [14,15] In conclusion, our results indicate that cell mediated immunity (CD3 +ve & CD68 +ve cells) appears to be more operational than humoral one in skin lesions with PR . References
1. Allen RA, Janniger CK and Schwartz RA (1995): Pityriasis rosea. Cutis 56 (4): 198-202.
2. Chuh A, Chan H and Zawar V (2004): Pityriasis rosea-evidence for and against an infectious aetiology. Epidemiol Infect 132 (3): 381-390.
3. Kempf W, Adams V, Kleinhans M, Burg G, Panizzon RG, Campadelli-Fiume G and Nestle FO (1999): Pityriasis rosea is not associated with human herpesvirus 7. Arch Dermatol 135 (9): 1070-1072.
4. Hsu S, Le EH and Khoshevis MR (2001): Differential diagnosis of annular lesions. Am Fam Physician 64 (2): 289-296.
5. Aiba S and Tagami H (1985): Immunohistologic studies in pityriasis rosea. Evidence for cellular immune reaction in the lesional epidermis. Arch Dermatol 121 (6): 761-765.
6. Hussein MR, Al-Badaiwy ZH and Guirguis MN (2004): Analysis of p53 and bcl-2 protein expression in the non-tumorigenic, pretumorigenic, and tumorigenic keratinocytic hyperproliferative lesions. J Cutan Pathol 31 (10): 643-651 .
7. Hussein MR and Wood GS (2002): Building bridges in cancer: mismatch repair and microstallite instability. Am J Dermatopathol 24 (1): 76-81.
8. Bos JD, van Garderen ID, Krieg SR and Poulter LW (1986): Different in situ distribution patterns of dendritic cells having Langerhans (T6+) and interdigitating (RFD1+) cell immunophenotype in psoriasis, atopic dermatitis, and other inflammatory dermatoses. J Invest Dermatol 87 (3): 358-361.
9. Bos JD, Hagenaars C, Das PK, Krieg SR, Voorn WJ and Kapsenberg ML (1989). Predominance of "memory" T cells (CD4+, CDw29+) over "naive" T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res 281(1): 24-30.
10. Sugiura, H., Miyauchi H and Uehara M (1988). Evolutionary changes of immunohistological characteristics of secondary lesions in pityriasis rosea. Arch Dermatol Res 280(7): 405-10.
11. Lugovic L, Lipozenocic J and Jakic-Razumovic J (2001): Atopic dermatitis: immunophenotyping of inflammatory cells in skin lesions. Int J Dermatol 40 (8): 489-494.
12. Yawalkar N, Hunger RE, Buri C, Schmid S, Egli F, Brand CU, Mueller C, Pichler WJ and Braathen LR (2001) a: A comparative study of the expression of cytotoxic proteins in allergic contact dermatitis and psoriasis: spongiotic skin lesions in allergic contact dermatitis are highly infiltrated by T cells expressing perforin and granzyme B. Am J Pathol 158 (3): 803-808.
13. Yawalkar N, Schmid S, Braathen LR and Pichler WJ (2001) b: Perforin and granzyme B may contribute to skin inflammation in atopic dermatitis and psoriasis. Br J Dermatol 144 (6): 1133-1139.
14. Bjerke JR (2002): The skin as an immunological organ. Tidsskr Nor Laegeforen 122 (8): 793-796.
15. Hussein MR (2005): Dendritic Cells and Melanoma Tumorigenesis: An Insight. Cancer Biol Ther 4 (5): 250-265.
© 2007 Egyptian Dermatology Online Journal |