|
|
Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces
apoptosis of many transformed but also of non-transformed cells. In addition,
TRAIL receptor activation has been reported to activate non-apoptotic signaling
pathways. In this study, 30 patients with atopic dermatitis, and 20, sex
and age matched healthy controls were enrolled. According to their response
to topical hydrocortisone cream, class 7and betamethasone valerate 0.1%
cream, class 3steroid therapy for 2 weeks, patients were divided into 2
groups, Group 1(good steroid responders) and Group II (poor steroid responders).
For every patient and control complete blood count using Coulter Counter,
serum total IgE quantitative measurement by a commercially available ELISA
kit, peripheral blood lymphocytes and monocytes TRAIL expression by direct
immunofluorescence flow cytometry were performed. We report an increased
expression of TRAIL in peripheral blood T cells and monocytes from patients
with atopic dermatitis (AD), group I &II, compared with control individuals.
The average absolute eosinophil count and IgE levels in group II atopic
dermatitis patients showed significant correlation with severity of the
disease and showed a non homogeneous distribution reflected by significant
association with family history of atopy, when both parents were atopic.
TRAIL expression in both CD4+ and CD8+ T cells as well as CD14+ monocytes
was significantly higher in group I AD patients compared with group II.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, highly pruritic, inflammatory
skin disease that frequently predates the development of allergic rhinitis
or asthma [1].
It is caused by complex interplay of the expression of many different genes
& multiple environmental factors affecting their expression [2].
In the dermis of AD lesions, there is a marked perivascular infiltrate in
which both CD4+ and CD8+ T cells are present .The majority of these cells
are of the CD45 Ro+ memory /effector phenotype and express the selective
skin-homing receptor, cutaneous lymphocyte -associated antigen (CLA) [3,4].
These T cells contain and release high amounts of preformed IL-5 and IL-13
[5]. In chronic
lesions, IFN-γ-producing cells have also been described [6].
Although Th2 type cytokines have been reported to be important for the development
of allergic diseases according to the so-called Th1/Th2 paradigm, this concept
is still controversial. As assessed by skin biopsy, both Th1and Th2 types
of inflammation are observed at the site of AD [7].
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a
member of the tumor necrosis factor (TNF) family with pro-apoptotic activity
[8]. Similar
to other members of the TNF family such as CD40L, FasL and TNF, TRAIL can
be released in soluble form and low amounts can be measured in sera of normal
controls [9].
Crystal structures have shown that, it occurs in trimer and it can be cleaved
by cysteine proteases to generate a soluble form of the ligand [10].
TRAIL is expressed on the surface of activated T lymphocytes, IFNα -stimulated
monocytes, dendritic cells and IFNγ -stimulated NK cells serving an anti-metastatic
and anti-tumorogenicity activity [11,12,13,14]
not only on malignant transformed cells but also of HIV-infected lymphocytes,
normal monocytes, neutrophils [15],
and macrophages [16].
Five TRAIL receptors have been identified: death receptor 4 (DR4/TRAIL-R1)
and death receptor 5 (DR5/TRAIL-R2) have the ability to initiate the apoptosis-signalling
cascade after ligation, whereas decoy receptor 1 (DcR1/TRID/TRAIL-R3) ,decoy
receptor 2 DcR2/TRAIL-R4/TRUNDD and the soluble receptor osteoprotegrin
lack this ability. They can afford protection from TRAIL- mediated killing
by being concomitantly expressed with the death receptors and competing
for binding to TRAIL, this may explain that TRAIL can selectively kill cancer
cells but not normal cells [17,18].
The decoy receptors, TRAIL-R3 and R4, are actually reported to prevent extensive
apoptosis in cells and tissues expressing both TRAIL and the death receptors,
TRAIL-R1 and R2. Osteoprotegerin is a soluble receptor for TRAIL and may
also act as a soluble decoy receptor. The balance of the expression levels
between the death receptors and decoy receptors is an important factor determining
the apoptotic effect of TRAIL [19].
TRAIL also has several physiological functions that are not limited to
the killing of transformed cells. TRAIL has been shown to induce apoptosis
in several primary cells, such as hepatocytes [20],
HIV-activated T cells [21],
plasma cells [22],
immature dendritic cells [23],
and neutrophils [15].
Moreover, TRAIL has been shown to activate a caspase-independent signaling
pathway leading to the activation of nuclear factor-kB (NF-kB) [24].
TRAIL also exerts anti-inflammatory activities, which may include the induction
of apoptosis in inflammatory cells [25],
blocking the cell cycle [26],
increasing the expression of interleukin 1 receptor antagonist (IL-Ra),
and activation of inhibitory phosphatases [27].
The ratio between IL-1 and IL-1Ra seems to be crucial for the intensity
of the inflammatory response in many diseases. Most studies involving the
physiological and pathological role of TRAIL were done in-vitro and little
is known about TRAIL expression in-vivo conditions [28].
Although the death of certain cells can lead to functional deficiencies,
prolonged survival of some effector cells can cause tissue injury and play
a role in the pathogenesis of diseases [29].
Recently, apoptosis of epidermal keratinocytes was highlighted as a mechanism
underlying the pathology of eczema in atopic dermatitis [4]
through three major mechanisms that have been postulated: increased IL5
expression by CLA+ T cells which extends the life span of eosinophils, upregulated
expression of Fas receptors and Fas L on peripheral blood CD4+ and CD8+
T cells of AD patients, lastly it was demonstrated that keratinocytes apoptosis
can be mediated by skin infiltrating T cells and is mediated by Fas L expressed
on the surface of T cells invading the epidermis or by soluble Fas L released
from peripheral blood lymphocytes of AD patients [30].
Also the damaged keratinocytes decrease the effectiveness of the epidermis
as a barrier against allergens and infectious agents and may contribute
to the development of chronic eczema [31].
Due to the structural and functional similarity between TRAIL and Fas L
and the fact that increased TRAIL expression on peripheral blood leukocytes
of AD patients has been reported [20],
TRAIL was assumed to play a role in the dysregulated apoptosis that contributes
to the pathogenesis of AD [32].
Aim of the work
The aim of this work was to examine the expression of TRAIL on peripheral
blood lymphocytes & monocytes of atopic dermatitis patients, to find its
correlation with disease severity before starting treatment with topical
steroids, and to asses the difference in the level of TRAIL expression between
those receiving steroids whether good or poor responders and those who are
not receiving steroids.
Patients and methods
Thirty patients with atopic dermatitis were enrolled in this study. These
patients were selected from the outpatient clinic of the dermatology, venereology
and andrology department, Ain Shams University, Cairo, Egypt.
Atopic dermatitis was diagnosed according to the criteria defined by
Hanifin J.M [33].
For every patient, detailed history was taken, including the age of onset,
duration of present illness, personal and/or family history of atopy. Exclusion
criteria included those with other allergic diseases such as asthma, allergic
rhinitis, or allergic conjunctivitis; also those with any other systemic
disease were excluded. Patients were asked to stop any systemic or topical
treatment for at least 4 weeks before enrollment in the study. A written
consent was obtained from every patient before the study was conducted.
Atopic dermatitis severity was assessed according to the objective SCORAD
scoring system, which was recommended by European Task Force on Atopic Dermatitis
(ETFAD )[35].
It is a modification of the SCORAD index that excludes the subjective symptoms
as pruritus and sleep loss, to minimize the errors caused by variability
in patients' ages and backgrounds. The objective SCORAD consists of the
extent and intensity items, the formula being A ?5 + 7B?2. In this formula
A is defined as the extent (0-100), and B is defined as the intensity (0-18).
The maximum objective SCORAD score is 83 (plus an additional 10 bonus
points). Bonus points are given for severe disfiguring eczema (on face and
hands). Based on its results, AD has been classified into mild (<15), moderate
(15-40) and severe (>40). The SCORAD index is influenced by subjective ratings
that may be affected by social and cultural factors. Therefore ETFAD [35]
recommends the objective SCORAD as it is representative and well evaluated.
Topical mild steroid therapy (hydrocortisone cream, class 7) [36]
was given for patients with mild atopic dermatitis whose objective SCORAD
was < 15. In addition, moderate steroid therapy (Betamethasone Valerate
0.1% cream, class 3) [36]
was given for patients with moderate and severe AD according to the objective
SCORAD index score. Topical steroid therapy was given for every patient
twice daily for for 7 days extended to 10 days according to the improvement..
According to their response to topical steroid therapy, patients were divided
into 2 groups. Group I (good responders): included 20 patients (8 males
and 12 females, with age range 10.5-19 years; mean ± SD 15.46 ± 4.3) 3(15%)
patients had mild AD, 11 (55%) had moderate AD and 6 (30%) had severe AD.
These patients showed clinical improvement with a shift in their objective
SCORAD to a lower score. Group II (poor responders): included 10 patients
(5 males and 5 females, with age range 10-18 years; mean ±SD 15.41 ± 4.6)
1(10%) patients had mild AD, 6(60%) had moderate AD and 3 (30%) had severe
AD. These patients did not respond to topical steroid therapy for 2 weeks,
or showed no shift in their objective SCORAD to a lower score). The control
group: composed of 20 sex and age matched healthy controls (11 males and
9 females, age range 11-19 years; mean ± SD 16±4.1), who had no personal
or family history of atopy or any other systemic disorder.
Venous blood was collected from all patients and controls included in
this study into tubes containing K- EDTA and analyzed before starting steroid
therapy.
Laboratory evaluation
For every patient and control the following laboratory tests were done:
Complete blood count using Coulter Counter (Coulter Microdiff 18, Fullerton,
CA, USA), Serum total IgE quantitative measurement by a commercially available
ELISA kit (Med' Biotech, Inc., Agenzyme Company, Industrial Road, San Carlos,
CA, USA),and Peripheral blood lymphocytes and monocytes analysis of the
patients and control groups was also performed by direct immunofluorescence
flow cytometry (Coulter EPICS XL),as follows: Venous blood was collected
into tubes containing K- EDTA (1.2mg\ml) and analyzed within 6 hours .One
hundred µl of each sample was stained using 10µl of each of FITC (fluorescin
isothiocyanate) conjugated mouse monoclonal antihuman CD14 antibodies (Caltag
Laboratories, CA, Burlingame), FITC conjugated mouse monoclonal antihuman
CD8 antibodies (Caltag Laboratories, CA, Burlingame), PC5 (phycoerythrincyanin)
conjugated mouse monoclonal antihuman CD4 antibodies (Caltag Laboratories,
CA, Burlingame), and PE (phycoerythrin) conjugated mouse monoclonal antihuman
TRAIL antibodies (R&D systems, Minneapolis, MN,USA ) .The tubes were then
incubated in dark at room temperature for 15 minutes . Erythrocytes were
lysed using ammonium chloride lysing solution (AI -Gomhoreya CA, Egypt ).After
two washes with phosphate buffered saline (PBS ),the cells were resuspended
in PBS for flow cytometric analysis ( fig 1).Negative isotype matched controls
( IGg mAB ) were included with each sample to determine the non specific
binding of the monoclonal antibodies. The results were expressed as the
percentage of the positive cells relative to the isotyic control (%), and
mean fluorescence intensity (MFI) which is defined as the ratio between
the mean fluorescence intensity of the cells incubated with the tested monoclonal
antibodies and mean fluorescence intensity of the cells incubated with isotypic
matched controls.
Statistical methodology
Analysis of data was done by IBM computer using SPSS ,version 12 as follows:
description of quantitative variables as mean, SD and range, and description
of qualitative variables as number and percentage, Chi-square test ,One-way
ANOVA test , Kruskall Wallis test , Unpaired t-test, Correlation co-efficient
test ,and ROC was used to find out the overall predictivity ,and the best
cut of value with detection of sensitivity, specificity at this cut off
value [36].
1 Sensitivity = true ve+/true +ve + false -ve
2 = ability of the test to detect +ve cases
3 specificity = true -ve/true-ve+ false +ve
4 = ability of the test to exclude negative cases
5 PPV(positive predictive value) = true+/true+ve +false +ve
6 = % of true +ve cases to all positive
7 NPV = true-/true-ve + false -ve
8 = % of the true -ve to all negative cases
P value >0.05 insignificant
P<0.05 significant
P<0.01 highly significant
Results
By comparing group I (steroid responders), and group II (non steroid
responders) as regards age of patients, sex, paternal and maternal family
history of atopy, our results show that in group II, 100% of patients had
positive (+) both paternal and maternal family history of atopy, while in
group I, all patients had + ve maternal family history of atopy and 50%
had +ve paternal family history of atopy. On the other hand there was no
statistically significant difference between group I and II as regards other
variables.
Also by comparing between age of disease onset and family history of
atopy, we found that by the presence of + F.H of atopy in both parents,
the age of disease onset was smaller (inverse correlation), with a statistically
significant difference between them (P<0.05).
According to the objective SCORAD results 4 (13%) patients had mild AD,
16 (53%) had moderate AD and 10 (33%) had severe AD. Comparison between
group I and II AD patients as regards different laboratory data (Hb, WBCs,
IgE, neutrophils and eosinophils), we found that group II had statistically
higher eosinophils and IgE levels than group I with a highly significant
difference between them (P<0.01). Also the average absolute eosinophil count
and IgE levels in group II patients of atopic dermatitis showed significant
correlation with severity of the disease and showed a non homogeneous distribution
reflected by significant association with family history of atopy, when
both parents were atopic. Both groups (I and II) had higher eosinophils
and IgE levels than the control group with also a highly statistically significant
difference between them (P<0.01).
Comparison between group I and II AD patients as regards the objective
SCORAD revealed no statistically significant difference between them.
Comparison between studied groups (I, II, and controls) as regards different
TRAILs, revealed that TRAIL expression in both CD4+ and CD8+ T lymphocytes
as well as CD14+ monocytes was significantly higher in AD patients (group
I and II) compared with normal controls, which usually demonstrated little
TRAIL expression (P<0.01). In particular, CD8+ T cells expressed large amounts
of TRAILs in AD. By comparing between group I and II, it was found that
group I had statistically higher levels of all TRAILs than group II (Table
1, Fig. 1, 2).
|
Variables |
Group I |
Group II |
Control |
F |
P |
|
N=20 |
N=10 |
N=20 |
|
CD8-MFI |
12.9+2.8 |
3.7+0.7 |
0.8+0.6 |
224 |
<0.01** |
|
(b, c) |
(a, c) |
(a, b) |
|
CD8 % |
69.9+10 |
69.9+9 |
1.2+1 |
467 |
<0.01** |
|
(c ) |
(c ) |
(a, b) |
|
CD4 MFI |
8.5+1.6 |
2.6+0.4 |
1+0.06 |
265 |
<0.01** |
|
(b,c) |
(a, c) |
(a, b) |
|
CD4 % |
49.5+11 |
50.9+10 |
18.4+10 |
48 |
<0.01** |
|
(c ) |
(c ) |
(a, b) |
|
CD14 MFI |
7+0.6 |
3.2+0.5 |
1.2+0.2 |
874 |
<0.01** |
|
(b, c) |
(a, c) |
(a, b) |
|
CD14 % |
54.5+14 |
52.7+14 |
11.9+5.2 |
73 |
<0.01** |
|
(c ) |
(c ) |
(a, b) |
Group I =a, Group II =b, Group III controls = c
Table (1): Comparison between studied groups as regards different
TRAILs.
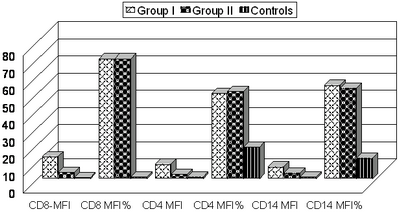
| Fig 1: Comparison between studied groups
as regards different TRAILs |
|
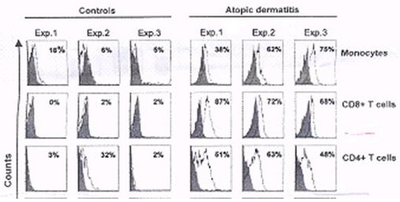
| Fig 2: Percentage of the positive cells relative
to the isotypic control: Expression of TRAIL in different
subsets of blood cells from AD patients and normal controls.
Monocytes, CD8+ cells, and CD4+ cells were analyzed
within PBMC and identified using anti-CD14, anti-CD8,
and anti-CD4 monoclonal antibody (mAb), respectively.
TRAIL expression was measured by flow cytometry using
mouse anti-TRAIL mAb. Isotypic Control IgG1 mAb staining
is shown in gray. |
|
By studying the correlation between TRAIL expression on CD4+ and CD8+
Lymphocytes and CD14+ monocytes with the objective SCORAD index score in
AD patients, it was found that all TRAILs expression were significantly
inversely correlated to objective SCORAD index score (i.e. increased TRAIL
expression was associated with a decrease in the objective SCORAD score,
P<0.01)(Fig 3).
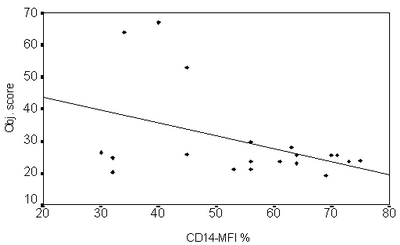
| Fig 3: Correlation between TRAIL expression
(e.g. CD 14 MFI) versus objective SCORAD index (Showing
an inverse correlation). |
|
Discussion
TRAIL is a member of the TNF superfamily, and has been implicated in
the regulation of various physiological and pathological immune responses
[14]. This
might be because of its wide expression among cells of the immune system,
including activated T cells [37],
B cells [38,29],
monocytes [28],
dendritic cells [39],
natural killer cells [37],
and neutrophils [28].
Most of these studies, however, were performed in vitro and little is known
about TRAIL expression under in vivo conditions.
TRAIL induces apoptosis in dendritic cells and neutrophils, in addition
to several primary cells and cancer cells, suggesting its critical role
in the regulation of the adaptive and innate immune responses [40].
It has been reported that apoptosis via TRAIL is important for homeostasis
of T cells [41]. TRAIL deficient mice showed increased susceptibility to
experimentally induced AD [42] and autoimmune arthritis [43]. On the other
hand, TRAIL application improved the clinical signs of experimental atopy.
Heishi et al 2002[44]
reported that apoptosis via TRAIL might be implicated in AD and that TRAIL
is expected to be a useful marker for evaluating AD. Patient phobia from
the steroid treatment is one of the obstacles that the physician can face
[45,46].
Heishi et al, 2002 [44]
reported that in vitro activated peripheral blood mononuclear cells (PBMC)
from AD patients with poor response to steroid, demonstrated low TRAIL expression
on their peripheral blood CD4+T and CD8+Tcells, compared to mononuclear
cells from AD patients with good response to steroid. That is why we aimed
to assess the in vivo difference in the level of TRAIL expression between
those receiving steroids whether good or poor responders and those who are
not receiving steroids.
The aim of this work was to examine the expression of TRAIL on peripheral
blood lymphocytes and monocytes of atopic dermatitis patients before starting
topical steroid therapy, to find its correlation with disease severity before
starting treatment with topical steroids, and to asses the difference in
the level of TRAIL expression between good steroid responders and poor steroid
responders.
Atopic dermatitis patients were classified into two groups according
to their response to. hydrocortisone cream, class 7and betamethasone valerate
0.1% cream, class 3 steroid therapy for 2 weeks Group 1 (good responding
group) improved within one week of treatment and group 2 (poorly responding
group) who did not or poorly responded after 2 weeks of treatment. Our results
showed a significant difference between group 1 and group 2 as regards family
history of atopy. In group II, 100% of patients had positive +ve both paternal
and maternal family history of atopy, while in group I, all patients had
+ve maternal family history of atopy and 50% had +ve paternal family history
of atopy. We also found that by the presence of +ve F.H of atopy in both
parents, the age of disease onset was smaller (inverse correlation), with
a statistically significant difference between them. Our results were in
agreement with Tay et al, 2002[47]
and Beltrani and Boguneiwicz, 2003[48],
who reported that the strongest risk factor is a parental history of atopy
or eczema, they also noted that maternal atopy is considered the most important
risk factor for the development of atopic disorders in offspring than paternal
atopy.
We found also that the average absolute eosinophil count and IgE levels
in group II patients of atopic dermatitis were significantly higher than
that of group I. Each of these parameters showed significant correlation
with severity of the disease and showed a nonhomogeneous distribution reflected
by significant association with family history of atopy, when both parents
were atopic. These findings were in agreement with the gene dose effect
postulated by many authors [49,50],
who suggested that subjects who inherit sets of atopy genes from both paternal
and maternal origin have higher levels of IgE and eosinophilic % than subjects
who inherit only one set of atopy genes either of paternal or maternal origin.
Comparison between studied groups (I, II, and controls) as regards different
TRAILs, revealed that TRAIL expression in both CD4+ and CD8+ T cells as
well as CD14+ monocytes was significantly higher in AD patients (group I
and II) compared with normal controls, which usually demonstrated little
TRAIL expression. In particular, CD8+ T cells expressed large amounts of
TRAILs in AD. Comparing group I and II, it was found that group I had statistically
higher levels of all TRAILs than group II, suggesting that TRAIL may exert
anti-inflammatory effects in AD the fact that was reported by many studies
[31,and 32]. Also TRAIL expression was inversely correlated with objective
SCORAD index (done before treatment). Our results confirm those of other
studies [32]
who also demonstrated increased TRAIL expression by several inflammatory
cells including peripheral blood T cells (CD4+T and CD8+Tcells), monocytes,
eosinophils, and neutrophils under in vivo conditions in AD. To our knowledge
there are no other reports on the TRAIL expression under in vivo inflammatory
conditions. The inverse correlation between TRAIL expression and objective
SCORAD, can be explained by the data suggesting that TRAIL expressing inflammatory
cells may contribute to the epidermal activation of IL-1 receptor antagonist
(IL-1Ra) in AD and that TRAIL might play an important role in pathogenesis
of AD [32].
To our knowledge this is the first report describing a -ve correlation between
TRAIL expression in AD patients' blood T cells and monocytes and disease
activity. The only study we could find regarding this point was done by
Heishi et al, 2002 [44]
who reported that in vitro activated PBMC from AD patients with poor response
to steroid, demonstrated low TRAIL expression on their peripheral blood
CD4+T and CD8+Tcells, compared to mononuclear cells from AD patients with
good response to steroid. Warnnissorn et al, 2003 [31]
examined expression of TRAIL in skin samples from AD patients. They found
significantly higher number of TRAIL-positive mononuclear cells in the lesions
of atopic dermatitis (mostly were CD68-positive macrophages) than in nonlesional
skin of atopic dermatitis, normal skin and psoriasis. This suggests that
TRAIL may also be involved in keratinocyte apoptosis in atopic dermatitis.
However, they could not exclude the possibility that TRAIL may have other
unknown functions in AD lesions.
To better understand TRAIL functions in AD, more studies should be performed
on blood and skin samples and controlled following pharmacological treatment,
as TRAIL may have other unknown functions in AD. Also more studies should
be done to determine the role of TRAIL in the regulation of the IL-1Ra/IL-1
system. More studies about TRAIL correlation with treatment response should
be done with greater number of patients and with different treatment options
to better understand the role of TRAIL.
References
1. Leung D.Y.M.: Atopic dermatitis: New insights and opportunities
for therapeutic intervention. J Allergy Clin Immunol; 105:860-76. , 2000
2. Leung DYM and Bieber T: Atopic dermatitis; Lancet; 361:151-159.
,2003
3. Akdis C.A., Akdis M., Simon D., et al : T cells and T
cell-derived cytokines as pathogenic factors in the non-allergic form of
atopic dermatitis. J Invest Dermatol; 113:628- 634, 1999
4. Trautmann A., Akdis M.,
Bröcker E., Blaser K. and Akdis
C.A.: New insights into the role of T cells in atopic dermatitis and allergic
contact dermatitis. TRENDS in Immunology; 22 (10): 530-2, 2001
5. Hamid Q., Naseer T., Minshall E.M., Song Y.L., Boguniewicz
M., and Leung D.Y.M.): In vivo expression of IL-12 and IL-13 in atopic dermatitis.
J Allergy Clin Immunol; 98:225-231, 1996
6. Trautmann A., Akdis M., Kleemann D. et al: T cell-mediated
Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous
dermatitis. J Clin Invest; 106:25-35, 2000
7. Novak N., Bieber T. And Leung D.Y.M.: Immune mechanisms
leading to atopic dermatitis. J Allergy Clin Immunol; 112:128-39. ,2003
8. Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang
C.P., Nicholl J.K., Sutherland G.R.et al: Identification and characterization
of a new member of the TNF family that induces apoptosis. Immunity; 3: 673-682.
. , 1995
9. Rus V., Zernetkinaa V., Puliaeva R., Cudricib C., Mathaia
S., and Viaa C.S.): Increased expression and release of functional tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) by T cells from
lupus patients with active disease. Clinical Immunology; 117: 48- 56. ,
2005
10. Griffith T.S. and Lyncht D.H): TRAIL: a molecule with
multiple receptors and control mechanisms. Current Opinion in Immunology;
10: 559-563. , 1998
11. Dorothee G.,Vergnon I., Menez J., Echchakir H., Grunenwald
D., Kubin M., Chouaib S., and Mami-Chouaib F.: Tumor-infiltrating CD4+ T
lymphocytes express APO2 ligand (APO2L)/TRAIL upon specific stimulation
with autologous lung carcinoma cells: role of IFN-alpha on APO2L/TRAIL expression
and -mediated cytotoxicity. J. Immunol; 169: 809- 817, 2002
12. Ehrlich S., Infante-Duarte C., Seeger B., and Zipp
F.: Regulation of soluble and surface-bound TRAIL in human T cells, B cells,
and monocytes. Cytokine; 24: 244- 253., 2003
13. Walczak H., Miller R.E., Ariail K., et al (2004): Tumoricidal
activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo.
Nat Med; 5:157-163.
14. Kimberley F.C. and Screaton G.R (2004): Following a
TRAIL: Update on a ligand and its five receptors. Cell Res; 14:359-372,
15. Matsuyama W., Yamamoto M., Higashimoto I., Oonakahara
K., Watanabe M., Machida K., et al. (2004): TNF-related apoptosis-inducing
ligand is involved in neutropenia of systemic lupus erythematosus, Blood
104:184-191.
16. Kaplan M.J., Ray D., Mo R.R., Yung R.L., and Richardson
B.C. (2000): TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T
cell killing of antigen-presenting macrophages. J. Immunol; 164:2897- 2904.
17. Kim J.M., Seol T.H., Esplen DW, Dorko JE, Billiar K,
and Strom TR: Apoptosis induced in normal human hepatocytes by tumor necrosis
factor-related apoptosis-inducing ligand. Nat Med; 6:564-567., 2002
18. Halaas O., Liabakk N.B., Vik R., Beninati C., Henneke
P., Sundan A., and Espevik T.: Monocytes stimulated with group B streptococci
or interferons release tumour necrosis factor-related apoptosis-inducing
ligand. Scand J Immunol; 60:74-81. ,2004
19. Kamohara H., Matsuyama W., Shimozato, O, ABE K., Galligan
C., Hashimoto, SI, Matsushima K and Yoshimura, T.: Regulation of tumpour
necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor
expression in human neutrophils. Immunology; 111:186-194., 2004
20. Zheng S.J., Wang P., Tsabary G., and Chen Y.H: Critical
roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest;
113:58-64, 2004
21. Miura Y., Misawa N., Maeda N., et al: Critical contribution
of tumor necrosis factor related apoptosis-inducing ligand (TRAIL) to apoptosis
of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J Exp Med;
193:651-660., 2001
22. Ursini-Siegel J., Zhang W., Altmeyer A., Hatada E.N.,
Do R.K., Yagita H., and Chen-Kiang S: TRAIL/Apo-2 ligand induces primary
plasma cell apoptosis. J Immunol; 169:4739-4744.,2002
23. Leverkus M., Walczak H., McLellan A., Fries H.W., Terbeck
G., Brocker E.B., and Kampgen E.: Maturation of dendritic cells leads to
up-regulation of cellular FLICE inhibitory protein and concomitant down-regulation
of death ligand mediated apoptosis. Blood; 96:2628-2631., 2002
24. Ehrhardt H., Fulda S., Schmid I., Hiscott J., Debatin
K.M., and Jeremias I.: TRAIL induced survival and proliferation in cancer
cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene;
22: 3842-3852. , 2003
25. Tecchio C., Huber V., Scapini P., et al: IFN alpha-stimulated
neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing
ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells.
Blood; 103:3837-3844.,2004
26. Song, K, Chen, Y, G?ke, R, et al: Tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation
and cell cycle progression. J Exp Med; 191:1095-1103.,2004
27. Daigle I., Yousefi S., Colonna M., Green D.R., and
Simon H.U.: Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic
signaling in neutrophils. Nat Med; 8:61-67., 2002
28. Tecchio C., Huber V., Scapini P., et al: IFN alpha-stimulated
eosinophils and CD4 + T cells release a soluble form of TNF-related apoptosis-inducing
ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on HIV infected
cells. Blood; 126:2830-2838. , 2007
29. Kemp T.J., Moore J.M., and Griffith T.S.: Human B cells
express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide
stimulation. J Immunol; 173:892-899.,2004
30. Lamhamedi-Cherradi S.E., Zheng S., Tisch R.M., and
Chen Y.H.: Critical roles of tumor necrosis factor-related apoptosis-inducing
ligand in type 1 diabetes. Diabetes; 52:2274-2278.,2003
31. Warnnissorn P. Nakao A. Suto H., Ushio H., Yamaguchi
N., Yagita H., Okumura K., and Ogawa H: Tumor necrosis factor-related apoptosis-inducing
ligand expression in atopic dermatitis. British Journal of Dermatology;
148 : 823-842.
32. Vassina E., Leverkus M., Yousefi S., Braathen L.R.,
Simon H.U., and Simon D.): Increased Expression and a Potential Anti-Inflammatory
Role of TRAIL in Atopic Dermatitis. J Invest Dermatol; 125:746 -752, 2005
33. Hanifin J.M., and Rajka G: Diagnostic features of atopic
dermatitis, Acta Derm Venereol. (Stockholm); 92: S44-S47., 2006
34. Oranje A.P., Glazenburg E.J., Wolkerstorfer A. and
Waard-van der Spek F.B.: Practical issues on interpretation of scoring atopic
dermatitis: the SCORAD index, objective SCORAD and the three-item severity
score. British Journal of Dermatology; 157 (4): 645-648.,2007
35. European Task Force on Atopic Dermatitis. Severity
scoring of atopic Dermatitis: the SCORAD Index (consensus report of the
European Task Force on Atopic Dermatitis). Dermatology; 186:23-31., 1993
36. Clinton Miller, Ph.D, and Rebecca G. Knapp: Clinical
epidemiology and biostatistics, published by Williams & Wilkins, Maryland:
3rd edition, 1994
37. Mirandola P., Ponti C., Gobbi G., et al: Activated
human NK and CD8+T cells express both TNF-related apoptosis-inducing ligand
(TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity.
Blood; 104: 2418-2424., 2004
38. Mariani S.M., and Krammer P.H.: Surface expression
of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur J Immunol 28:1492-1498.,1998
39. Fanger N.A., Maliszewski C.R., Schooley K., and Griffith
T.S. : Human dendritic cells mediate cellular apoptosis via tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL). J Exp Med; 190:1155-1164.,
1999
40-Goodwin RG and Smith CA: The TRAIL of death. Apoptosis; 3(8): 83-88, 1998.
41-Chou A.H., Tsai H.F., Lin L.L., Hsieh S.L., and Hsu P.I.: Enhanced
proliferation and increased IFN- gamma production in T cells by signal transduced
through TNF-related apoptosis inducing ligand. J Immunol; 167:1347-52.,
2001
42-Lamhamedi-Cherradi S.E., Zheng S.J., Maguschak K.A., Peschon J.,
and Chen Y.H.: Defective thymocyte apoptosis and accelerated autoimmune
diseases in TRAIL/ mice. Nat Immunol; 4:255-260.,2003
43-Lamhamedi-Cherradi
S.E., Zheng S., Tisch R.M., and Chen Y.H.: Critical roles of tumor necrosis
factor-related apoptosis-inducing ligand in type 1 diabetes. Diabetes; 52:2274-2278.,
2003
44-Heishi, M, Kagaya, S, Katsunuma, T, et al. :High-density oligonucleotide
array analysis of mRNA transcripts in peripheral blood cells of severe atopic
dermatitis patients. Int Arch Allergy Immunol;129:57-66.,2005
45-Tilles
G., Wallach D., and
Taıeb
A: Topical therapy of atopic dermatitis: Controversies
from Hippocrates to topical immunomodulators. J Am Acad Dermatol; 56:295-301.,
2005
46-Buys L.M., Pharm.D, and B.C.P.S.: Treatment Options for Atopic Dermatitis.
Am FAM Physician; 75:523-8, 530, 2007
br>47-Tay Y.K., Kong K.H., Khoo L.,
Goh C.L., and Giam Y.C.: The prevalence and descriptive epidemiology of
atopic dermatitis in Singapore school children. Br J Dermatol; 146 (1):
101-6.,2006
48-Beltrani V.S., and Boguneiwicz M: Atopic Dermatitis; Dermatology
Online Journal 9(2):1,2003
49-Vercelli D: Genetics, epigenetics, and the
environment ,Switching, buffering, releasing. J Allergy Clin Immunol;113:381-6.,2004
50-Staumont-Sallé D. Dombrowicz D., Capron M and Delaporte E.: Eosinophils
and urticaria. Vlinical reviews in allergy and immunology; 30:13-18 ,2006
© 2007 Egyptian Dermatology Online Journal
|



