|
|
Abstract:
Psoriasis, a form of over-active wound healing
response, is relatively common, chronic, inflammatory and hypersensitive
disease of unsolved pathogenesis affecting skin and joints in 2-3% of the
general population. At the cellular level, psoriasis is characterized by
markedly increased epidermal proliferation and incomplete differentiation,
elongation, dilatation and "leakiness" of the superficial plexus of dermal
capillaries, and a mixed inflammatory and immune cell infiltrate of the
epidermis and papillary dermis. Psoriasis is a skin disease driven by immune
system which starts below the skin's surface and cause severe pain and
adverse mental health effects. Our present study is an attempt to compile
all most of the possible protein targets known so far for the treatment of
psoriasis. The identified proteins are STAT3, Want5, Endothelin-1, enzyme -
alpha secretase, S100 proteins, p53, Serum Response Factor, HSP70 and Bcl-x.
Introduction
Psoriasis comprises red, scaly patches of skin, which
usually have very well defined edges, appear covered by silvery flaky
surface [1].
These patches, which are sometimes referred to as plaques, usually itch or
feel sore. The redness is explained by impressive growth and dilation of
superficial blood vessels [2].
They most often occur on the elbows, knees, other parts of legs, scalp,
lower back, face, palms, and soles of the feet, but they can occur on skin
anywhere on the body [3].
The disease may also affect the fingernails, the toenails, and the soft
tissues of the genitals and inside the mouth [4].
It is often symmetrical, affecting both sides of the body. Psoriasis is
often so mild it is barely noticed by the affected person, but it can
occasionally be so severe that the patient must be admitted to hospital for
treatment. The disease occurs in all age groups, primarily affecting adults.
The most common ages for psoriasis to first appear are in the late teens and
in the 50s. It affects men and women equally, although in children, girls
are more commonly affected than boys [5].
Being characterized as dermatological disease, psoriasis is a non-
contagious lifelong skin disease and also not an outcome of poor hygiene.
Unfortunately it is often overlooked or dismissed because it is not
typically life threatening [6].
Depending on the variability in morphology,
distribution, severity and cause, they are divided into different types.
Some can occur independently or at the same time as other variants, or one
may follow another. Most common is the plaque psoriasis which starts off in
small areas, then gradually enlarge and develop thick, dry plaque. They
usually appear symmetrically, that is, in the same areas on opposite sides
of the body. Eventually separate patches may join together to form larger
areas as the disorder develop. In some cases, the patches can become very
large and cover wide areas of the back or chest (known as geographic plaques
because they resemble maps). Plaque psoriasis persists for long periods.
Gutate psoriasis, from the Greek word "gutta" meaning droplet, includes
teardrop-shaped patches 1-10 mm in diameter, usually distributed on the
trunk and often on the extremities but can also occur on the head [7].
Gutate psoriasis can also develop in patients who have already had another
form of psoriasis. Pustular psoriasis is characterized by white, sterile
pustules surrounded by red skin. It tends to follow a cycle-reddening of the
skin followed by formation of pustules and scaling. The pus consists of
white blood cells. There are two types of pustular psoriasis, generalized
pustular psoriasis (von Zumbusch) and palmo-plantar pustulosis (PPP).
Generalized pustular psoriasis is rare and represents active, unstable
disease. This form is very widespread and the eruptions often occur in
repeated waves lasting days or weeks. PPP causes pustules on the palms and
soles; it is uncertain whether it really is a form of psoriasis [8].
Inverse psoriasis is morphologically distinct from
traditional plaques. It affects the flexures, particularly the armpits, the
groin and under the breasts. Flexural lesions are devoid of scales and
appear as red, shiny, well demarcated plaques. Erythroderma is a scaling,
itching, inflammatory process that involves all or almost the entire body
surface. It may either arise from chronic plaque which progresses, becoming
confluent and extensive, or it may be a manifestation of unstable psoriasis
precipitated by infection, drugs, stress or withdrawal of corticosteroids.
Erythrodermic psoriasis can impair thermoregulation of the skin, leading to
hypothermia; it can change metabolism due to loss of keratin, iron and folic
acid during the profuse scaling and it can cause edema, especially around
the ankles. Nail psoriasis is seen in 40-45 % of skin psoriasis patients;
the commonest finding is small pits in the nail plate. The nail may also
detach from the bed at its distal or lateral attachments, known as
onycholysis. Psoriatic nails also often exhibit yellow-brown discoloration
and are deformed and thickened [9].
Psoriatic arthritis is a joint disease core common among patients with
arithiritis [10].
Other types of psoriasis include flexural psoriasis; characterized by smooth
well-defined patches in body folds, scalp psoriasis; one or more scaly
plaques in the scalp, sebopsoriasis, which is characterized by an overlap of
seborrheic dermatitis and psoriasis, affects scalp, face, ears and chest,
intraoral psoriasis which includes desquamation inside the mouth, most often
associated with the more severe forms of cutaneous psoriasis, Koebnerised
psoriasis which arises in healing wounds or scars and photosensitive
psoriasis affecting sun-exposed skin.
Targets for Psoriasis
Signal Transducer and Activator of Transcription 3
(STAT3): [fig1]
It is a protein involved in transmitting extracellular
signals to the nucleus, is crucial to the development of the skin disease
psoriasis [11].
STAT proteins transmit signals from cytokines or growth factors that have
cell-surface receptors associated with tyrosine kinase activity. Kinases,
such as members of the Janus kinase family or SRC family, phosphorylate
these receptors and provide docking sites for inactive STAT monomers, which
are in turn phosphorylated and form activated dimers. Activated STATS move
to the nucleus and are involved in regulating many genes that control
fundamental biological process including apoptosis, cell proliferation and
immune responses [12].
Blocking the function of STAT3 using antisense oligo-nucleotides inhibited
the onset of, and reversed, established psoriatic lesions. Further analysis
revealed a dual requirement of both activated STAT3 in keratinocytes as well
as in T cells, indicating that the pathogenesis of psoriasis is rooted in a
co-operative process involving STAT3-regulated genes in both skin cells and
the immune system [13].
Another study revealed that phosphatyrosyl peptides block STAT3-mediated DNA
binding activity, gene regulation and cell transformation. To identify small
molecule inhibitors of STAT3 the ability of STAT3 SH2 domain binding peptide
PY*LKTK (Y* represents phosphorylation) to disrupt STAT3 activity in vitro
has been investigated [14].
The presence of PY*LKTK, but not PYLKTK or PFLKTK, in nuclear extracts
results in significant reduction in the levels of DNA binding activities of
STAT3.
Wnt5a:
It is a member of the wingless-type pattering
regulators important in pre-natal development. Wnt5 and its receptors fzd3,
fzd5 as well as fzd6 are restricted to specific layers in normal epidermis,
analogous to their zonal distribution in hair follicles, suggesting a role
in adult skin differentiation [fig.2]. Wnt5a and fzd5 are both over
expressed and re-distributed in the epidermis of psoriasis which involves
disturbed keratinocytes differentiation. Functionally, Wnt5a lowers the
concentration of IFN required to induce target genes, and increases the
magnitude of IFN target gene induction, suggesting a molecular mechanism
underlying IFN hypersensitivity in psoriasis [15].
The marked over expression of want5a and Fzd5 in psoriasis suggests that
this ligand receptor pair may actively derive the chronic inflammatory and
hyper- proliferative nature of this phenotype. This is supported by large
number of evidences. First, Wnt5a induces IL-12 [16],
which is central to psoriasis as indicated by therapeutic efficacy of
antibodies targeted at IL-12 p40 and by the genetic association of IL-12
with psoriasis [17,18].
Second, Wnt5a itself is strongly induced by STAT3 [19],
thereby acting as a potential effectors molecule for psoriasis like skin
disease seen in STAT3 over-expressing transgenic mice [20].
Wnt5a induces epithelial differentiation and stimulate endothelial cells
proliferation; both are important elements in psoriasis. Wnt5a is
unregulated in wound healing [21]
and psoriasis lesions can classically be triggered by skin wounding. Wnt5a
derived from endothelial signals through Fzd5 to induce the translocation of
NFAT and subsequent induction of IL-2 in T-cells [22].
Wnt5a signaling in psoriasis may also be related to the activation of the
nuclear hormone receptor. PPARδ is identified as central mediator in
psoriasis [15].
Thus, Wnt5a may synergize with IFNα to induce the expression of PPARδ in
activated T-lymphocytes [23].
Moreover, PPARδ stimulates keratinocytes proliferation and in several
biological processes acts by antagonizing another PPARγ [24].
Wnt5a also represses PPARγ trans-activation, thereby synergizing with PPARδ
functionally [25].
Conversely, PPARγ inhibits STAT3 [26]
which, when over expressed, induces Wnt5a and causes a psoriasis like
phenotype in vivo [20].
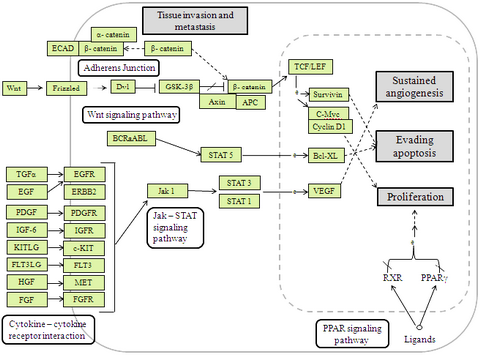 | Fig 1:
Signaling pathway, which includes STAT3, Wnt, Bcl-XL and PPAR
γ. |
|
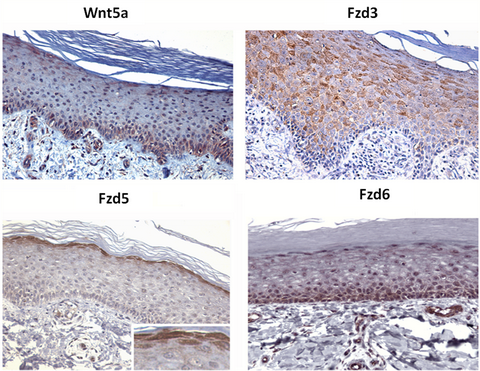
|
Fig 2:
Expression of Wnt5a, Fzd3, Fzd5, and Fzd6 in adult human
epidermis. Immuno histochemistry of paraffin-embedded skin [15].
[Courtesy: Malgorzata Romanowska et al]. |
|
Endothelin-1:
It could be one of the targets of psoriasis [27].
Endothelin (ET)-1 produced in keratinocytes acts through ETA receptor as an
autocrine growth factor for these cells [28].
ETs are a family of three vasoactive peptides, termed ET-1, ET-2 and ET-3,
which induce their biological actions through at least two major receptor
subtypes that belong to the family of G-protein coupled receptors: a
selective ETA receptor, which binds ET-1 and ET-2 with high affinity and
ET-3 with low affinity, and a non-selective ETB receptor which binds all ET
isopeptides with equal affinity. It has recently been reported that
compounds that antagonize the action of ET-1 by blocking the ETA receptor
may control the growth of tumor cells in which the ET-1 autocrine loop is up
regulated [29,30].
High levels of ET-1 have been found in psoriatic skin and in the serum of
patients with psoriasis [31,32].
Inflammatory cytokines such as interleukin-1a increase the production of
ET-1 that in turn may lead to the chronic stimulation of keratinocytes
proliferation.
Enzyme blockers:
They could also be new psoriasis treatment. The
keratinocytes produce an enzyme - alpha secretase - which cuts the APP down
to size as sAPPa. After adding inhibitors, the team observed that the
discharge of sAPPa was almost completely arrested in the cells of psoriasis
patients. As a result, the greatly increased division rate of the
keratinocytes dropped back to normal values, a reduction of 50 to 60 per
cent [33].
S100 proteins:
They are calcium activated signaling proteins that
interact with target proteins to modulate biological processes [34].
There are 18 identified S100 family proteins, thirteen S100 protein gene are
located within the epidermal differentiation complex on human chromosome
1q21 [35].
Although eight S100 proteins are expressed in human epidermis [36],
in psoriatic tissues S100A7, S100A8, S100A9 are markedly over expressed [37].
p53:
It is a phosphor-protein,
which shows transcription factor-like properties [38],
plays a central role in cell cycle regulation mechanisms and cell
proliferation control. p53 protein acts as a "guardian" of the genome - its
activation protects the organism from tumorgenesis. DNA damaging agents
induce the activity of this protein, which leads to cell cycle arrest in the
G1 or G2 phase and in the case of ineffective DNA repair apoptosis, is
initiated [39].
Many genes, particularly oncogenes and tumor suppressor genes, could be
involved in the deregulation of the cell-cycle (increased cell division),
which is probably important in the development of psoriatic lesions [40,41].
In lesional psoriatic skin the count of p53 positive cells was significantly
higher than in the skin samples taken from healthy individuals. p53 positive
cells were located most commonly in the basal layer of the epidermis of both
healthy skin and non-lesional psoriatic skin. In lesional psoriatic skin p53
positive cells were present in all layers of the epidermis [fig3] [42].
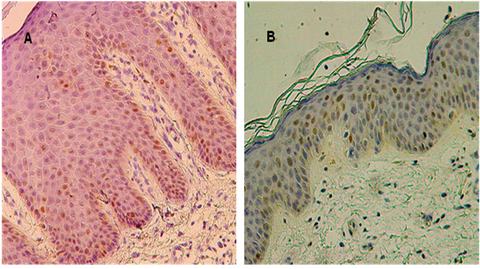 |
Fig 3: p53 immuno-reactivity: A: Lesional psoriatic skin (x200),
B: Non-lesional skin taken from psoriatic patient (x200) [42],
[Courtesy: W. Baran et al] |
|
Protein SRF (Serum Response Factor):
It produced/ expressed in large amounts in normal
healthy human skin cells (keratinocytes) and it maintains skin regulation.
In psoriatic or wounded skin, SRF's are expressed at low levels. Without
sufficient SRF, skin becomes dead i.e. skin divides excessively and no
longer able to develop normally. As a result of their deficiency, the
skeleton of the skin cells is disturbed and destroyed. The cells lose
contact with their neighbours and the matrix that surrounds them.
Consequently, the outer layer of skin loses its compact layering. Fissures
form which enables water to evaporate more easily and the skin, dries out
more quickly, thus making the intermediate spaces more susceptible to
foreign bodies and bacteria. This in turn triggers an inflammatory reaction
that induces the skin cells to divide and impairs their differentiation.
Psoriasis patients lack SRF almost entirely. Triggers/factors that cause the
down-regulation of SRF are still unknown. The suspects include other
proteins, such as cytokines or other transcription factors [43].
Heat Shock Protein 70 (HSP70):
HSP represent a highly
conserved class of molecules that increase during cellular stress response
to a wide variety of stimuli. Based on molecular mass and sequence homology,
HSPs are classified into families including ubiquitin, small HSPs of 20 to
28KDa, HSP60, HSP70 and HSP90. Antigenic sites of many pathogenic HSPs,
particularly primary target for B cell and T cell responses. In the HSP70
family are heat shocking cognate protein 70 (HSC70), which constitutively
expressed during normal cell development and differentiation and the major
heat or stress inducible protein HSP70 [fig4]. They are nuclear and
cytoplasmic proteins exhibiting approximately 97% sequence homology and
forming stable complex in stressed cells. HSP27, HSP60 and HSP70 and their
ligands common HSP receptor CD91 have been reported to be increased in
lesional psoriatic skin [44].
 | Fig
4: A: Normal skin: light HSP70 immuno-staining in
basal epidermal cell layer with negative immune-reactivity in
supra-basal and superficial epidermal layers (immunoperoxidase
X200), B: Psoriasis vulgaris: intense HSP70 immuno-staining in basal and supra-basal epidermal cell layers with light superficial layer immuno-staining and dermal inflammatory infiltrate immune reactivity (immunoperoxidase X200). [44], [Courtesy: Amina Gamal el Din et al]. |
|
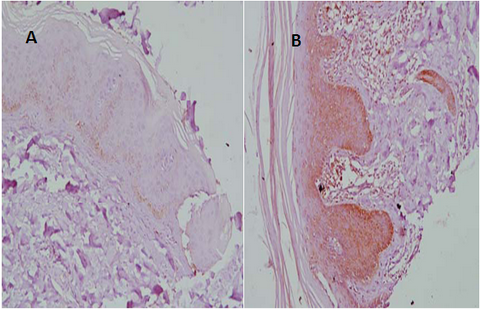
Bcl-2 family proteins: Increased expression of Bcl-x protein is associated with psoriatic epidermal hyperplasia [fig5]. Strong Bax and Bak expression in involved psoriatic skin may probably have inhibitory mechanisms counteracting intensive proliferation [45].
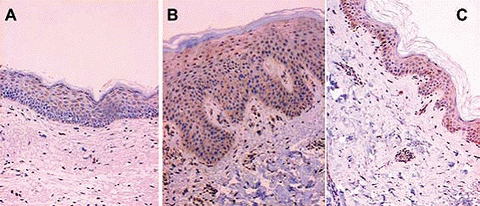 | Fig
5:
Immuno-histochemical staining for Bcl-X. A: Bcl-X positive cells in
upper granular layers of normal skin (× 200). B: Strong diffuse Bcl-X
staining in involved psoriatic skin (× 200). C: Intense diffuses Bcl-X
staining in uninvolved psoriatic skin (× 200) [45],
[Courtesy: Tanja Batinac et al]. |
|
Discussion
In this review, we have tried to extract the possible
protein targets for psoriasis. As per the literature survey, all above
discussed proteins i.e. STAT3, Want5, Endothelin-1, enzyme - alpha secretase,
S100 proteins, p53, Serum Response Factor, HSP70 and Bcl-x have potential to
serve as target for the treatment of psoriasis. By extensive review of
number of research papers it seems that these proteins can play an important
role in development of drug molecules which can prevent, mitigate and cure
psoriasis.
References
1. Mehta V, Malhotra SK. Psychiatric Evaluation of Patients with Psoriasis Vulgaris and Chronic Urticaria. German J Psychiatry 2007; 10: 104- 110
2. James TE, Rajan PD, Sun WG, Tilo H, Christophers E, Voorhees JJ. The Genetics of Psoriasis. Arch Dermatol 1994; 130 (2): 216- 224
3. Friberg C. Genetic studies of psoriasis and psoriatic arthritis, Goteborg University, Sweden 2007.
4. Questions and answers about psoriasis. NIH Publication 2003
5. Psoriasis treatment. DermNet NZ 2008
6. Fowler JF, Duh MS, Rovba L, Buteau S, Pinheiro L, Lobo F, Sung J, Doyle JJ, Swensen A, Mallett DA, Kosicki G. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol 2008; 59 (5): 772- 780
7. Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol 1992; 128 (1): 39- 42
8. Ameen KM et al., Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol 2003; 120 (4): 627- 632
9. Anhorn KA et al. HLA antigens in psoriatic arthritis. J Rheumatol 1986; 13 (3): 586- 592
10. Andrea LN, Steven BP, Joel MG. The epidemiology of psoriasis. Expert Rev. Dermatol 2006; 1 (1): 63- 75
11. Yu H, Jove R. The STAT of cancer: new molecular targets come of age. Nature Rev. cancer 2004; 4: 97- 105
12. O'Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immune-suppression: targeting The JAK/STAT pathway. Nature Rev. Drug Discov 2004; 3: 555- 564
13. Gattlieb AB, Psoriasis: emerging therapeutic strategies. Nature Rev. Drug Discov. 2005; 4: 19-34
14. Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001; 30; 276(48), 45443- 45455
15. Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, Foerster J. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis and synergizes with Type1 interferon. PloS ONE 2009; 4 (4): 53- 54
16. Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006; 108: 965- 973
17. Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R et al. A large scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 2007; 80: 273-290
18. Langley RG, Leonardi C, Yeilding N, Guzzo C et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. Krueger GG. N Engl J Med 2007; 356: 580- 592
19. Katoh M, Katoh M. STAT3-induced WNT5A signaling loop in embryonic stem cells, adult normal tissues, chronic persistent inflammation, rheumatoid arthritis and cancer. Int J Mol Med 2007; 19: 273- 278
20. Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 2005; 11: 43-49
21. Fathke C, Wilson L, Shah K, Kim B, Hocking A et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 2006; 7: 4
22. Murphy LL, Hughes CC. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. J Immunol 2002; 169: 3717- 3725
23. Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt5A/ frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum 2001; 44: 772-781
24. Romanowska M, Yacoub N, Seidel H, Donandt S, Gerken H et al. PPARdelta enhances keratinocyte proliferation in psoriasis and induces heparinbinding EGF-like growth factor. Invest Dermatol 2008; 128: 110- 124
25. Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 2007; 9: 1273- 1285
26. Wang LH, Yang XY, Zhang X, Huang J, Hou J et al. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity 2004; 20: 205-218
27. Simeone P, Teson M, Latini A, Carducci M, Venuti A. Endothelin-1 could be one of the targets of psoriasis therapy. Br J Dermatol 2004; 151: 1272- 1288
28. Bagnato A, Venuti A, Di Castro V et al. Identification of the ETA receptor subtype that mediates endothelin induced autocrine proliferation of normal human keratinocytes. Biochem Biophys Res Commun 1995; 209: 80- 86
29. Venuti A, Salani D, Manni V et al. Expression of endothelin 1 and endothelin A receptor in HPV-associated cervical carcinoma: new potential targets for anticancer therapy. FASEB J 2000; 14: 2277- 2283
30. Bagnato A, Cirilli A, Salani D et al. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res 2002; 62: 6381- 6384
31. Bonifati C, Mussi A, Carducci M et al. Serum endothelin 1 levels are increased in psoriatic patients and correlate with disease severityInt. J Immunopath Ph 1997; 10: 81- 82
32. Bonifati C, Mussi A, Carducci M et al. Endothelin-1 levels are increased in sera and lesional skin extracts of psoriatic patients and correlate with disease severity. Acta Derm Venereol (Stockh) 1998; 78: 22- 26
33. [ http://www.drugresearcher.com/Emerging-targets/Enzyme-blockers-could-be-new-psoriasis-treatment]
34. Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B et al. Psoriasin: a novel chemotactic protein. J Invest Dermatol 1996; 107: 5- 10
35. Engelkamp D, Schafer BW, Mattei MG, Erne P, Heizmann CW. Six S100 genes are clustered on human chromosome 1q21: identification of two genes coding for the two previously unreported calcium-binding proteins S100D and S100E. Proc Natl Acad Sci 1993; 90: 6547-6551
36. Boni R, Burg G, Doguoglu A, Ilg EC, Schafer BW, Muller B et al. Immunohistochemical localization of the Ca2+ binding S100 proteins in normal human skin and melanocytic lesions. Br J Dermatol 1999; 137: 39- 43
37. Broom AM, Ryan D, Eckert RL. S100 Protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem 2003; 51(5): 675- 685
38. Soini Y, Kamel D, Paakko P et al. Aberrant accumulation of p53 associates with Ki67 and mitotic count in benign skin lesions. Br J Dermatol 1994; 131: 514- 520
39. Batinac T, Gruber F, Lipozencic J et al. Protein p53 - structure, function and possible therapeutic implications. Acta Dermatovenerol Croat 2003; 11: 225- 230
40. Bishop JM. Molecular themes in oncogenesis. Cell 1991; 64: 235- 248
41. Marshall CJ. Tumor suppressor genes. Cell 1991; 64: 313- 326
42. Baran W, Szepietowski JC, Szybejko-Machaj G. Expression of p53 protein in psoriasis. Acta Dermatoven APA 2005; 14(3): 79- 83
43. Sabine Werner and colleagues. The Protein SRF Keeps The Skin Healthy. Science Daily Apr.2009
44. Abdel HM, Abdel Fattah NS, Saleh HM, Gamal el-Din AA. Evaluation of heat shock protein 70 (HSP70) in Psoriasis Vulgaris: Expression and Correlation with SA. PALD 2007; 18 (3): 103- 104
45. Batinac
T, Zamolo G, Hadzisejdić I, Zauhar G, Brumini G, Ruzić A, Persić V.
Expression of Bcl-2 Family proteins in Psoriasis. Croat Med J 2007; 48: 319-
326 © 2010 Egyptian Dermatology Online Journal |





