|
|
Abstract:
Background and Objectives: Vitiligo is a fairly
common disorder with tremendous psycho-social impact. The association of
vitiligo with a number of autoimmune diseases especially involving the
thyroid gland, is of considerable interest since early detection can lead to
proper management of patients. We aim at studying the thyroid profile and
the presence of anti-thyroid antibodies in patients with vitiligo.
Methods: One hundred and ninety two patients attending the
outpatient department were included in this case controlled study. Thyroid
profile (free T3, free T4 and TSH) and ELISA for anti-TPO antibodies was
done in cases as well as controls. Results:
Thirty one patients (16.1%) with vitiligo had an abnormal thyroid profile (2
were hyperthyroid and the rest hypothyroid). Twenty one patients (11%) were
positive for anti-TPO antibodies as against 5 (2.6%) controls (p
value=0.001). Conclusion: Patients with vitiligo
have an increased incidence of anti-TPO antibodies. Female patients are
affected to a greater extent. Introduction:
Vitiligo is a primary, acquired disorder characterized by the presence of
well-circumscribed, milky-white or chalk-white macules on the skin and
mucous membranes as a result of loss of functioning melanocytes from the
involved areas. Hair overlying a vitiligo lesion may also become white (leucotrichia)
[1-4].
Vitiligo is relatively common, incidence varying from 1-2%. All races are
affected and both sexes are affected equally. Vitiligo is a fairly common
skin disorder in the Kashmir valley. The valley of Kashmir, known to its
inhabitants as 'Kashir', is situated in the extreme north of India and is
perched securely among the Himalayas at an average height of about 6000 feet
above sea level. The prevalence of vitiligo has been found to be about 2.3%
in patients attending the outpatient department in Kashmir [5].
Vitiligo can be classified into generalized and localized types. Vitiligo
vulgaris, acrofacial and vitiligo universalis are included in the
generalized type and segmental and focal vitiligo in the localised type.
Vitiligo is also classified as segmental, non-segmental and mixed types. The
segmental type is localized to a segment of the integument; the segment
might be composed of several, or parts of several adjacent dermatomes, or
have no relation to dermatomes at all. It does not generally cross the
midline and shows a relatively stable course after its early rapid-spreading
phase. The non-segmental type presents as bilateral, usually symmetrical
macules [6-8].
Numerous studies have demonstrated the significant increase in frequency of
various autoimmune disorders including autoimmune thyroid disease,
pernicious anaemia, Addison's disease and systemic lupus erythematosus in
patients with vitiligo [9-11].
Organ specific antibodies to thyroid (anti-thyroid peroxidase, anti-thyroglobulin),
gastric parietal cell, and adrenal tissue are found more frequently in the
serum of patients with vitiligo than in the general population [12-17].
Various thyroid antibodies including thyroid stimulating antibody, anti-thyroglobulin
antibody and anti-thyroid peroxidase antibody (TPO) have been detected in
patients with vitiligo at an increased frequency [18-22].
These antibodies are detectable in autoimmune thyroid disorders, anti-TPO
being the most sensitive for the diagnosis and follow-up for these
disorders. Antithyroid peroxidase antibody, historically referred to as the
anti-microsomal antibody is an established, sensitive tool for the detection
of early subclinical autoimmune thyroid disease. Thyroid peroxidase enzyme
is involved in thyroid hormone synthesis. After iodine enters the thyroid
gland, it is trapped and oxidized in an organification reaction that
involves thyroid peroxidase (TPO) and hydrogen peroxide [23].
This study was designed keeping in mind the high prevalence of vitiligo in
our state, to assess associated thyroid disease in vitiligo. Material And
Methods:
The study was conducted on 192 patients with vitiligo and an equal number
of age and sex matched controls. The cases were recruited from the
out-patient wing of the department of Dermatology, STD and Leprosy, SMHS
hospital (associated teaching hospital of Government Medical College,
Srinagar). The inclusion criteria included patients in the 6-60 years age
group with vitiligo, without any associated medical or cutaneous disease
related to the thyroid gland directly or indirectly. Children less than 6
years of age, patients with known thyroid disease on replacement therapy,
thyroid surgery and those on anti-thyroid medication, and patients with
other causes of leukoderma were excluded. A complete medical history
especially information pertaining to vitiligo like age at onset, duration of
disease, family history, any was taken from each patient. Age of onset was
defined as the age at which the first spot was noticed. In each patient a
thorough general physical and systemic examination including examination of
the thyroid gland was done. A detailed cutaneous examination was done to
determine the site of involvement, morphology, type and the percentage of
body surface area involved by vitiligo. A note was made of the hair
involvement and mucosal involvement. Diagnostic criteria for vitiligo were
those of the Vitiligo European Task Force [24].
The extent of involvement was determined by the "Wallace rule of nines".
Clinical photographs were taken in selected patients. Controls were
selected among patients in the age group of 6-60 years who attended the
out-patient department for minor unrelated dermatological problem. Children
less than 6 years of age and patients with known thyroid disorders or
autoimmune disorders were not included in the controls. Apart from routine
investigations, thyroid function tests (free T3, freeT4 and TSH) were done
by electro-chemiluminesense assay (ECLIA) in both cases and controls.
Subclinical hypothyroidism was diagnosed on the basis of a raised TSH and a
normal T3 and T4 values, the diagnosis of overt hypothyroidism required a
low T3 and T4 as well. The diagnosis of subclinical hyperthyroidism was
based on a low TSH and a normal T3 and T4; overt hyperthyroidism was
diagnosed by a low TSH and a raised T3 and T4 value. The following values
were taken as normal:
TSH 0.2-4.3 µIU/ml
fT3 0.8-2.1 ng/ml
fT4 5-14.1 ng/dl
Anti-thyroid peroxidase (anti-TPO) antibodies were determined by means of
Microplate Enzyme Immunoassay using Accubind Elisa Microwells (Monobind Inc
USA). Values in excess of 40 IU were considered to be positive.
Statistical methods: The statistical analysis of the data was done
using student's t-test for difference of means and chi-square test. These
tests were referenced for p values and a p value less than 0.05 was taken as
significant. Fischer's exact test was also used. The analysis of the data
was performed by using SPSS computer program (Statistical Package for Social
Sciences, SPSS Inc. Chicago, USA) version 10.0. Results:
One hundred ninety two (192) patients with vitiligo in the age range of
6-60 years were studied. The study group comprised of 101 females and 91
males. The mean age in females was 19.61±11.054 years and the mean age in
males was 20.89±9.909 years. Generalized type of vitiligo was present in 140
(72.9%) patients and localized vitiligo was present in 52 patients (27.1%).
Of these, 134 (69.8%) patients had vitiligo vulgaris, 28 (14.6%) had focal
vitiligo, 24 (12.5%) had segmental vitiligo, 5 (2.6%) had acrofacial
vitiligo and 1 (0.5%) patient had vitiligo universalis. Classical
presentation of vitiligo was seen in 177 (92.2%) patients; followed by
trichrome vitiligo in 15 (7.8%) patients. Cases of quadrichrome vitiligo,
inflammatory vitiligo or confetti-like macules were not seen in any of the
patients included in our study. The age of onset of vitiligo varied from
6-60 years. In 172 (89.3%) patients with vitiligo, age of onset was ? 30
years. The mean age of onset was 17.58±9.811 years for females and
19.03±9.215 years for males. The mean age of onset in males and females was
not significantly different. Onset before 30 years of age was seen in a
statistically significant number of patients (p < 0.0001). Involvement
of the mucosa was seen in 6 (3.1%) patients of which oral mucosa was
involved in 5 (2.6%) and genital mucosa in 1 (0.5%) patient. Among patients
with mucosal involvement, 5 (2.6%) had generalized vitiligo and 1 (0.5%) had
localized vitiligo. Leucotrichia was found in 34 (17.7%) patients. Of these
27 (14.1%) had generalized vitiligo and 7 (3.6%) had localized vitiligo. The
difference in hair involvement in localized and generalized types of
vitiligo was not significant (p = 0.67). Koebner phenomenon was seen in 23
(11.9%) patients out of which 9 (4.6%) were males and 14 (7.3%) females. All
these cases had generalized vitiligo. A family history of vitiligo was
present in 34 (17.7 %) patients of whom 26 (13.5%) had generalized vitiligo
and 8 (4.2%) had localized vitiligo. No difference in the family history was
seen in males and females (p = 0.666). Only 30 patients (15.6%) with
vitiligo had a stable course, of whom 11 had generalized vitiligo and 19 had
localized vitiligo. 162 patients (84.4%) had an unstable course.
Results of thyroid assessment in the study group: Among patients with
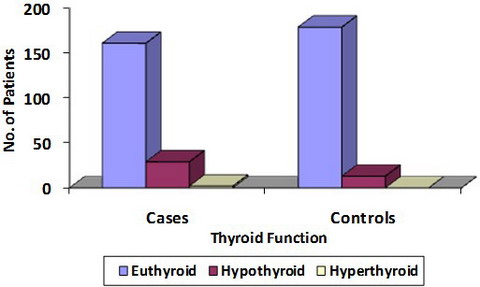
vitiligo, 161 (84%) were found to be euthyroid, 29 (15%) were hypothyroid
and 2 (1%) were hyperthyroid. The difference in thyroid profile between
generalized and localized types was not significant (p = 0.761). Among the
29 hypothyroid patients, 22 were females and 7 were males. The higher number
of females with thyroid dysfunction was statistically significant (p =
0.008). In the hypothyroid group, 24 (83%) patients had subclinical
hypothyroidism and 5 (17%) patients had clinical hypothyroidism. Anti-TPO
antibodies were present in 21 (11%) patients of vitiligo. Of these, 17 (81%)
had generalized vitiligo and 4 (29%) had localized vitiligo. Nineteen
(90.5%) patients were females and 2 (9.5%) patients were males. The higher
incidence in females was statistically significant (p < 0.0001). Out of the
21 patients with anti-TPO antibody 18 (86%) had early onset vitiligo. No
association was found between age of onset and the presence of anti-TPO
antibody (p = 0.602). Seventeen (81%) patients with a positive anti-TPO
antibody had vitiligo vulgaris, 2 (9.5%) had segmental and 2 (9.5%) had
focal vitiligo. The difference among the various types was found to be
non-significant (p = 0.506). Mucosal involvement was not seen in any patient
with a positive anti-TPO antibody. Five (24%) of the 21 patients with
positive anti-TPO antibodies had leukotrichia. The association was not
statistically significant (p = 0.438). Out of the 21 patients with positive
anti-TPO antibodies, 4 (19%) patients had a positive family history.
Eighteen (86%) of the patients with positive anti-TPO antibodies were
hypothyroid and 3 (14%) patients were euthyroid (Table 1). The
presence of anti-TPO antibodies had a significant association with the
thyroid profile of the patient (p < 0.0001). The control group comprised
of 101 females and 91 males. The mean age in the control group was
20.42±9.604 years.
|
Anti-TPO Antibody |
Thyroid Function |
Total |
|
Hypothyroid |
Euthyroid |
Hyperthyroid |
|
Absent |
11 |
158 |
2 |
171 |
|
6.40% |
92.30% |
1.30% |
100.00% |
|
Present |
18 |
3 |
0 |
21 |
|
85.70% |
14.30% |
0% |
100.00% |
|
Total |
29 |
161 |
2 |
192 |
|
15.10% |
83.90% |
1.00% |
100.00% |
|
χ2
=
91.687 |
p value < 0.0001
(Significant) |
Table 1: Anti-tpo Antibody And Thyroid Profile In Vitiligo
Comparison between cases and controls: One hundred and sixty two
(83.9%) cases with vitiligo were euthyroid, 29 (15.1%) were hypothyroid and
2 (1%) were hyperthyroid. Among the controls 179 (93.2%) were euthyroid and
13 (6.8%) were hypothyroid (Table 2). The difference between thyroid
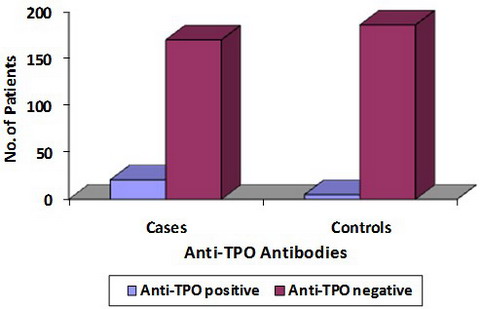
profile in cases and controls was not significant (p = 0.011). Anti-TPO
antibody was positive in 21 (11%) of the cases and 5 (2.6%) controls
(Table 3). The association between the presence of anti-TPO antibody and
vitiligo was statistically significant (p = 0.001).
|
Group |
Thyroid Function |
Total |
|
Hypothyroid |
Euthyroid |
Hyperthyroid |
|
Cases |
29 |
161 |
2 |
192 |
|
15.10% |
83.90% |
1.00% |
100.00% |
|
Controls |
13 |
179 |
0 |
192 |
|
6.80% |
93.20% |
0.00% |
100.00% |
|
Total |
42 |
340 |
2 |
384 |
|
10.90% |
88.50% |
0.50% |
100.00% |
|
χ2
= 9.048 |
p value = 0.011 (NS) |
Table 2: Comparison Of Thyroid Function In Cases And Controls
|
Group |
Anti-TPO antibody |
Total |
|
Negative |
Positive |
|
Cases |
171 |
21 |
192 |
|
89% |
11% |
100.00% |
|
Controls |
187 |
5 |
192 |
|
97.40% |
2.60% |
100.00% |
|
Total |
358 |
26 |
384 |
|
93.20% |
6.80% |
100.00% |
|
χ2
=10.56 |
p value = 0.001 (Significant) |
Fischer’s exact test 0.002 |
Table
3: Comparison of Anti-TPO Antibody among cases and controls
Discussion And Conclusions:
Vitiligo is known to be associated with a number of systemic disorders
most of them of autoimmune aetiology; whether these occur prior to or after
the onset of vitiligo is not known. Our study was planned to evaluate the
association of thyroid disease and presence of anti-TPO antibody in patients
with vitiligo keeping in mind that both vitiligo and thyroid disorders are
fairly common in this part of the world and no studies of this kind, to the
best of our knowledge, have been done previously in our population. About
5 to 15% of euthyroid women and up to 2% men have thyroid antibodies and
such individuals are at an increased risk of developing thyroid dysfunction.
Almost all patients with autoimmune hypothyroidism and up to 80% of those
with Graves' disease, have TPO antibodies, usually at high levels [23].
Most of the studies available on this subject have shown a significant
association between thyroid disease especially anti-thyroid antibodies and
vitiligo [18-22].
Not many studies pertaining to this subcontinent are available. The largest
study from India involving 1436 patients did not reveal any significant
association between thyroid disorders and vitiligo [25].
In our study of 192 patients with vitiligo 31 (16.1%) patients, had an
abnormal thyroid function of which 24 (77%) were females. 1% patients were
hyperthyroid and the majority 29 (15%) were hypothyroid. Among the
hypothyroid patients, 24 (83%) had subclinical disease. No difference was
observed in the thyroid profile among patients with generalized or localised
vitiligo (p = 0.761). Our study did not reveal a significant difference in
the thyroid profile in cases and control (p = 0.011). This could in part be
due to the fairly high prevalence of iodine deficiency disorders in our
state accounting for the high level of thyroid dysfunction among controls.
Anti-thyroid peroxidase antibodies (anti-TPO) were detected in a
statistically significant 21 (11%) patients with vitiligo (p = 0.001). This
number, even though significant, is lower than that in studies involving
mostly Caucasians. The significance of this is as yet, unknown. It could be
due to a number of unknown confounding factors in our population which needs
further evaluation. The presence of anti-thyroid antibodies in various
studies ranges from 7 to 58% of vitiligo patients (17% in the large study on
Caucasians by Alkhateeb et al), in studies from the developed world. Studies
from India reveal a very low percentage (0.5%) of patients with vitiligo,
have thyroid disease. Even though it has been attributed to lower detection
rates in this part of the world, an actual difference between the two
populations in the prevalence of autoimmune disorders could also be
contributor [25]. Our study has
demonstrated an increased incidence of anti-thyroid antibodies in patients
with vitiligo. This is in concordance with the results obtained in various
studies [25-41].
Among the 21 patients with positive anti-TPO antibodies, 18 (86%) patients
had hypothyroidism and about 3 (14%) patients had no detectable abnormality
in their routine thyroid function. It has been suggested that if anti-TPO
antibodies are present with a normal thyroid function, thyroid
ultrasonography should be carried out to detect any changes compatible with
autoimmune thyroiditis. This helps detect subclinical autoimmune disease, so
that monitoring and possible replacement can be done. If anti-TPO antibody
is positive along with increased TSH levels (after two tests 4 weeks apart)
patient should be referred for an endocrinology evaluation as these patients
have a high risk of progression to clinical hypothyroidism. In fact it has
been proposed that patients with vitiligo should be annually screened for
thyroid function (TSH, anti-TPO antibody, anti-thyroglobulin antibody [34].
Most of our patients with a positive anti-TPO antibody 17 (81%) had
generalized vitiligo and 4 (19%) had localized vitiligo. The difference
between the two types of vitiligo was not significant (p = 0.38). Hair
involvement was seen in 24% and none of the patients with a positive anti-TPO
antibody had mucosal involvement. In our study, females were found to have
higher incidence of anti-TPO antibody positivity. Of the 21 patients with a
positive anti-TPO antibody 90% were females. This is in conformity with the
observation that autoimmune disease is more common in females [23].
A family history of vitiligo was seen 19% patients with anti-TPO antibody,
however this was not found to be significant (p = 0.865). Among the anti-TPO
antibody positive patients, 85.7% patients had developed vitiligo before the
age of 30 years but since the majority of patients develop vitiligo before
30 years of age this association was not found to be significant (p =
0.602). Morgan et al., [29] in his study
found auto-antibodies were especially raised in generalized vitiligo. In
another study, patients with late onset and higher mean age were found to
have a higher incidence of anti-TPO antibodies [14].
Various other studies have described the association of long lasting
vitiligo, mucosal involvement or early onset vitiligo with the presence of
anti-TPO antibodies [12-23].
No such association between the presence of anti-TPO antibody and any of
these factors was found in our study. Our findings have important
implications for evaluation and surveillance of patients with vitiligo. As
is corroborated by other studies autoimmune thyroid screening should become
standard medical practice in patients with vitiligo for early detection and
appropriate management. References
1.
Ortonne JP, Bahadoran P, Fitzpatrick TB, Moscher DB, Hori Y. Hypomelanoses
and hypermelanoses. In Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith
LA, Katz SI., eds, Fitzpatrick's dermatology in general medicine, 6th ed.,
McGraw Hill Co: New York 2003; 1: 839- 847
2. Lee SJ,
Cho SB, Hann SK. Classification of vitiligo. In Gupta S, Olsson MJ, Kanwar
AJ, Ortonne JP., eds, Surgical management of vitiligo Blackwell Publishing
Delhi 2007; 3: 20- 29
3. Bleehan SS, Anstey AV.
Disorders of skin colour. In: Burns T, Breathnach S, Cox N, Griffiths C. eds
Rook's Textbook of Dermatology, 7th ed., Blackwell Science: UK 2004; 2:
39.53-39.57
4. Spielvogel RL, Kantor GR. Pigmentary
disorders of the skin. In Elder DE, Elenitsas R, Johnson BL, Murphy GF. eds,
Lever's histopathology of the skin, 9th ed., Lippincott Williams and Wilkins
Philadelphia 2005:705- 711
5. Masood Q, Hassan I.
Pattern of skin disorder in Kashmir valley. Indian J Dermatol 2002; 47(3):
147- 148
6. Grimes PE: White patches and bruised
souls: Advances in the pathogenesis and treatment of vitiligo. J Am Acad
Dermatol 2004; 51(1): 55- 57
7. Fargnoli MC, Bolognia
JL. Pentachrome vitiligo. J Am Acad Dermatol 1995; 33: 853- 856
8. Koga M, Tango T. Clinical features and course of type A and type B
vitiligo. Br J Dermatol 1988; 118: 223- 228
9. Dawber
RP Clinical associations of vitiligo. Postgrad Med J 1970; 46(535): 276- 277
10. Gopal KV, Ramarao GR, Kumar YH, Appa Rao MV, Vasudev P, Srikant.
Vitiligo: a part of systemic autoimmune process. Indian J Dermatol Venereol
Leprol 2007; 73(3): 162- 165
11. Kovacs SO.
Vitiligo. J Am Acad Dermatol 1998; 38(5): 647-666.
12. Bystryn JC. Theories in the pathogenesis of depigmentation: Immune
hypothesis. In Hann SK, Norlund JJ eds. Vitiligo. Blackwell Science, London,
2000: 21
13. Ongenae K, Van Geel N, Naeyaert JM.
Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res 2003;
16(2): 90-100
14. Brostoff J. Autoantibodies in
patients with vitiligo. Lancet 1969; 2(7613): 177- 178
15. Kemp EH. Autoantibodies as diagnostic and predictive markers of
vitiligo. Autoimmunity 2004; 37: 287- 290
16. Grimes
PE, Halder RM, Jones C, Chakrabarti SG, Enterline J, Minus HR, Kenney JA.
Autoantibodies and their clinical significance in a black vitiligo
population. Arch Dermatol 1983; 119(4): 300- 303
17.
Rezaei N, Gavalas N, Weetman A, Kemp E.Autoimmunity as an aetiological
factor in vitiligo. J Eur Acad Dermatol Venereol 2007; 21(7): 865- 876
18. Cunliffe WJ, Hall R, Newell DJ, Stevenson CJ. Vitiligo, thyroid disease
and autoimmunity. Br J Dermatol 1968; 80(3): 135- 139
19. Kurtev A, Dourmishev AL. Thyroid function and autoimmunity in children
and adolescents with vitiligo. J Eur Acad Dermatol Venereol 2004; 18: 109-
111
20. Pal SK, Ghosh KK, Banerjee PK. Thyroid
function in vitiligo. Clin Chim Acta 1980; 106(3): 331- 332
21. Ai J, Leonhardt MJ, Heymann RW. Autoimmune thyroid disease. Etiology,
pathogenesis and dermatological manifestations. J Am Acad Dermatol 2003; 48:
641- 659
22. Koppers LE, Palumbo PJ. Pigmentation
and the endocrinologist. Med Clin North Am 1972; 56(4): 1041- 1049
23. Jameson JL, Weetman AP. Disorders of the thyroid gland. In Braunwald E,
Fauci AS, Kasper DL, Hauser SL, Longo DL and Jameson JL. eds Harrison's
Textbook of Internal Medicine 15th ed McGraw Hill New York 2001; 2: 2060-
2078
24. Taieb A, Picardo M, VETF Members. The
definition and assessment of vitiligo: a consensus report of the Vitiligo
European Task Force. Pigment Cell Res 2007; 20(1): 27- 35
25. Handa S, Kaur I. Vitiligo: clinical findings in 1436 patients. J
Dermatol 1999; 26(10): 653- 657
26. Alkhateeb A,
Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and
associated autoimmune diseases in Caucasian probands and their families.
Pigment Cell Res 2003; 2003: 208- 214
27. Hegedüs L,
Heidenheim M, Gervil M, Hjalgrim H, H?ier- Madsen M. High frequency of
thyroid dysfunction in patients with vitiligo. Acta Derm Venereol 1994;
74(2): 120- 123
28. Kumar V, Shankar V, Chaudhary S,
Bhatia KK, Mehta LK, Arora N, Arora DR. Radio-active iodine uptake in
vitiligo. J Dermatol 1990; 17(1): 41- 43
29. Morgan
M, Castells A, Ramirez A. Autoantibodies in vitiligo: clinical significance.
Med Cutan Ibero Lat Am 1986; 14: 139- 142
30.
Betterle C, Caretto A, De Zio A, Pedini B, Veller-Fornasa C, Cecchetto A,
Accordi F, Peserico A. Incidence and significance of organ-specific
autoimmune disorders (clinical, latent or only autoantibodies) in patients
with vitiligo. Dermatologica 1985; 171(6): 419- 423
31. Boisseau-Garsaud AM, Garsaud P, Calès-Quist D, Hélénon R, Quénéhervé C,
Claire RC. Epidemiology of vitiligo in the French West Indies (Isle of
Martinique). Int J Dermatol 2000; 39(1): 18- 20
32.
Mandry RC, Ortiz LJ, Lugo-Somolinos A, Sanchez JL. Organ specific antibodies
in vitiligo patients and their relatives. Int J Dermatol 1996; 35: 18- 21
33. Iacovelli P, Sinagra JL, Vidolin AP, Marenda S, Capitanio B, Leone G et
al. Relevance of thyroiditis and of other autoimmune diseases in children
with vitiligo. Dermatology 2005; 210: 26- 30
34.
Kakourou T, Kanaka-Gatenbein C, Papadopoulou A, Kaloumenou E, Chrousos GP.
Increased prevalence of chronic autoimmune thyroiditis in children and
adolescents with vitiligo. J Am Acad Dermatol 2005; 53(2): 220- 223
35. Daneshpazhooh M, Mostofizadeh M, Javad Behjati, Akhyani M, Robati R.
Antithyroid peroxidase antibody and vitiligo: a controlled study.BMC
Dermatol 2006; 6: 3
36. Birlea SA, Fain PR, Spritz
RA. A Romanian population isolate with high frequency of vitiligo and
associated autoimmune diseases. Arch Dermatol 2008; 144(3): 310- 316
37. Laberge G, Mailloux CM, Gowan K, Holland P, Bennett DC, Fain PR and
Spritz RA. Early disease onset and increased risk of other autoimmune
diseases in familial generalized vitiligo. Pigment Cell Res 18; 300- 305
38. Zettinig A, Tanew A, Fischer G, Mayr W, Dudczak R and Weissel M.
Autoimmune diseases in vitiligo: do anti-nuclear antibodies decrease thyroid
volume. Clin Exp Immunol 2003; 131: 347- 354
39.
Shong YK, Kim JA. Vitiligo in autoimmune thyroid disease. Thyroidology 1991;
3: 89- 91
40. Korkij W, Soltani K, Simjee S,
Marcincin PG, Chuang TY. Tissue-specific autoantibodies and autoimmune
disorders in vitiligo and alopecia areata: a retrospective study. J Cutan
Pathol 1984; 11(6): 522- 530
41. Dave S,D'Souza M,
Thappa DM, Reddy KS, Bobby Z. High frequency of thyroid dysfunction in
Indian patients with vitiligo. Indian J Dermatol 2003; 48: 68- 72
© 2010 Egyptian Dermatology Online Journal |