|
|
Abstract
Dermatophytes can digest keratin and other
proteinaceous substrates present in skin and its appendages such as nail,
hair, and feather and use it as its sole source of carbon and nitrogen.
Proteolytic and keratinolytic activities of dermatophytes have been a
subject of interest for several years to understand the pathogenicity of
infection. In this study we intend to elucidate the keratinase activity
profile among the three ecological groups viz. geophilic, zoophilic and
anthrophophilic of dermatophytes. Six isolates of each species of T. rubrum
and E. floccosum, M. gypseum and M. canis were grown on mineral medium
consist of human hair, human nail and chicken feather individually. The
keratinolytic activity was measured spectrophotometrically at 660 nm
following Folin-Ciocalteu method. The data collected was analyzed using
ANOVA followed Duncan Multiple Range test. A statistically significant
difference (P= 0.000**) was noted in the enzyme activity of different
ecological groups of dermatophytes. T. rubrum and E. floccosum (anthrophophilic
deramtophytes) recorded the lowest activity for all the substrates when
compared to the geophilic (M. gypesum) and zoophilic (M. canis)
counterparts. We presume that the enzyme moderation could be an attribute
for obligate Anthropization in certain dermatophytes.
Introduction
Keratins are the most abundant proteins in epithelial
cells of vertebrates and represent the major constituent of skin and its
appendages such as nail, hair, feather and wool. Keratin is a stable
insoluble protein with rigid structure due to the presence of several
cross-linking disulfide bonds. Dermatophytes can digest keratin and other
proteinaceous substrates present in the skin, nail, hair etc. and use it as
its sole source of carbon and nitrogen [1].
Proteolytic and keratinolytic activities of
dermatophytes have been a subject of interest for several years to
understand the pathogenicity of infection [2].
Dermatophytes of different ecological groups have evolved owing to their
differences in host specificity. However a detailed comparative study of the
selective preference of the different native keratin substrates among the
ecological groups of dermatophytes and its possible co-relation to
pathogenicity is lacking. Therefore in the current study we intended to
elucidate the difference in the keratinase activity of Trichophyton rubrum &
Epidermophyton floccosum (Anthrophophilic) Microsporum gypseum (Geophilic)
and Microsporum canis (Zoophilic) on keratin substrates such as human hair,
human nail and chicken feather.
Materials And Methods
Six isolates each of T. rubrum and E. floccosum
(clinical origin), M. gypseum
(2 clinical, 4 soil origin) and M. canis (from pet
dogs) grown on Sabouraud Dextrose broth were used in the current study.
Twenty micro liters of the fungal spore suspension of the dermatophytes viz.
T. rubrum, E. floccosum, M. of MgSO4.7H2O, 0.1g of
Cacl2.2H2O, pH 7.5 gypseum, and M. canis prepared in
distilled water and adjusted to an absorbance of 0.6 at 450 nm were used as
inoculums for the present study. Nine sets of 10 ml mineral medium (g/l of
0.5g KCl, 1.0g K2HPO4, 0.5g MgSO4, 2.0g
NaNO3, 0.01g FeSO4.7H2O, 30g Dextrose) were
prepared in 25 ml conical flasks containing 1% of different native keratin
substrates viz. human hair, human nail and chicken feather individually for
each isolate of each species. The above substrates viz. human hair, human
nail and chicken feather were washed with ethanol, dried, and hammer milled
individually prior to addition to the medium. The inoculated conical flasks
were incubated on a rotary shaker (130rpm) at room temperature. From 4th day
of incubation one flask (1/9) per strain per substrate was taken for
keratinase enzyme assay. Keratinase assay was done on every alternate day
from 4th day of incubation till 20th day [3].
One and half ml of culture was transferred to
Eppendorf tube and centrifuged at 5000 rpm for 10 minutes. The culture
supernatant (crude enzyme extract) was used for enzyme assay. A known volume
(500ul) of the supernatant was incubated with 1% (w/v) keratin powder
(Hi-media, India) in 20 mM Tris-HCl buffer (pH 8.0) at 30°C for 5h. The
reaction was terminated with 6% (w/v) Tri Chloro Acetic acid (TCA) and then
allowed to stand for 30min followed by centrifugation at 15,000xg for 10min.
The resultant supernatant was mixed with 1:1 dilution of Folin-Ciocalteu
reagent and 0.5 M NaOH and then incubated at room temperature for 1hour. The
keratinolytic activity was measured spectrophotometrically (PerkinElmer) at
660 nm. The keratinolytic activity was expressed in keratin units (KU), and
1KU is defined as an increase of 0.01 OD at 660 nm in 1h [3,4].
The data collected was analyzed using ANOVA followed Duncan Multiple Range
test.
Results
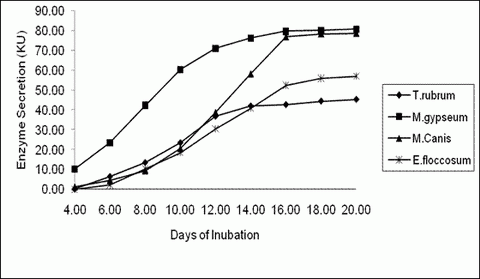
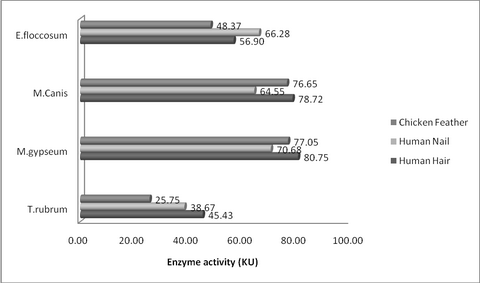
Human Hair: M. gypseum recorded maximum keratinase
activity (80.75KU) which was closely followed by M. canis (78.72KU) on the
20th day. E. floccosum recorded relatively lower activity of 56.90KU, while
T. rubrum recorded the least activity of 45.43KU on the 20th day. (P value =
0.01**) (Table 1, 2 & fig 1, 4)
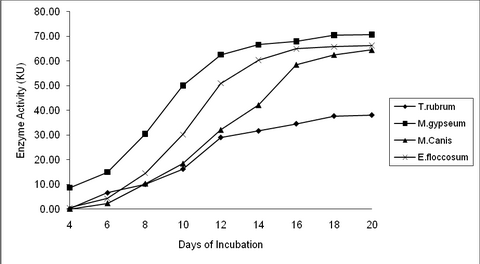
Human Nail: T. rubrum recorded the least keratinase
activity of 38.07KU on the 20th day, while M. gypseum recorded the maximum
activity of 70.68KU. M. canis and E. floccosum recorded moderate activity of
64.55 and 66.28 KU respectively for the human nail on the 20th day (P value
= 0.01**). (Table 1, 2 & fig. 2, 4)
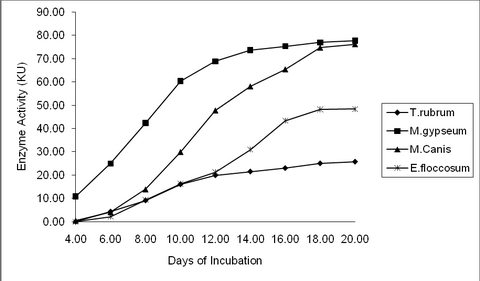
Chicken Feather: M. gypseum (77.65KU) and M. canis
(76.20KU) recorded higher keratinase activity, while that of E. floccosum is
moderate (48.37KU) on the 20th day. T. rubrum recorded the lowest (25.75KU)
keratinase activity among the tested organisms on the 20th Day (P value =
0.01**). (Table 1, 2 & fig. 3, 4)
|
Days |
Human Hair |
Human Nail |
Chicken Feather |
|
Fungal Strains |
Fungal Strains |
Fungal Strains |
|
T. |
M. |
M. |
E. |
T. |
M. |
M. |
E. |
T. |
M. |
M. |
E. |
|
rubrum |
gypseum |
Canis |
floccosum |
rubrum |
gypseum |
Canis |
floccosum |
rubrum |
gypseum |
Canis |
floccosum |
|
4 |
0.18 |
10.25 |
1.15 |
0 |
0.13 |
8.62 |
0 |
0.47 |
0 |
10.9 |
0.52 |
0 |
|
6 |
6.47 |
23.55 |
4.4 |
2.3 |
6.62 |
14.93 |
2.32 |
4.28 |
4.37 |
24.97 |
4.12 |
2.17 |
|
8 |
13.53 |
42.37 |
9.38 |
10.33 |
10.1 |
30.45 |
10.25 |
14.52 |
9.12 |
42.37 |
13.92 |
9.37 |
|
10 |
23.52 |
60.45 |
20.53 |
18.57 |
16.23 |
50.05 |
18.4 |
30.15 |
16.05 |
60.4 |
29.97 |
16.3 |
|
12 |
36.88 |
71.12 |
38.75 |
30.48 |
29.03 |
62.57 |
32.08 |
51 |
19.9 |
68.87 |
47.77 |
21.28 |
|
14 |
42.1 |
76.33 |
58.23 |
40.83 |
31.65 |
66.63 |
42.17 |
60.4 |
21.53 |
73.68 |
58.1 |
30.95 |
|
16 |
42.8 |
79.67 |
76.97 |
52.35 |
34.55 |
67.98 |
58.58 |
64.92 |
23 |
75.28 |
65.42 |
43.37 |
|
18 |
44.6 |
80.22 |
78.52 |
55.92 |
37.68 |
70.48 |
62.58 |
65.82 |
25.03 |
76.95 |
74.7 |
48.2 |
|
20 |
45.43 |
80.75 |
78.72 |
56.9 |
38.07 |
70.68 |
64.55 |
66.28 |
25.75 |
77.65 |
76.2 |
48.37 |
Table 1: Keratinase Activity (KU) by dermatophytes at different time interval in response to keratin substrates
|
Substrate |
T. rubrum |
M. gypseum |
M. canis |
E. floccosum |
|
|
Mean (KU) |
SD |
Mean (KU) |
SD |
Mean (KU) |
SD |
Mean (KU) |
SD |
p-value |
|
Human Hair |
45.43a |
1.89 |
80.75d |
0.95 |
78.72c |
0.73 |
56.90b |
1.09 |
0.01** |
|
Human Nail |
38.07a |
2.55 |
70.68c |
1.79 |
64.55b |
2.73 |
66.28b |
1.28 |
0.01** |
|
Chicken
Feather |
25.75a |
2.11 |
77.65d |
1.22 |
76.20c |
1.44 |
48.37b |
0.44 |
0.01** |
Table 2: Peak Keratinase Activity of dermatophytes on different substrates
** Denotes Significant at 1% level.
Different Alphabets (a/b/c/d) between the organisms denotes significant above 5% level.
The Order of alphabet denotes lower to higher enzyme activity between organisms
 | Fig 1:
Keratinase Activity by dermatophytes at different time interval in
response to Human Hair |
|
 | Fig
2:
Keratinase Activity by dermatophytes at different time interval in
response to Human Nail |
|
 | Fig
3:
Keratinase Activity by dermatophytes at different time interval in
response to Chicken Feather |
|
 | Fig
4:
Peak Keratinase Activity (KU) of dermatophytes on different
substrates |
|
Discussion
Among the three keratinaceous sources viz. human hair,
human nail and chicken feather, human hair was the most preferred keratin
source for isolates of T. rubrum, M. gypseum and M. canis as all these
organisms showed maximum keratinase activity for human hair. Among these
three organisms it was also observed that M. gypseum started the keratinase
secretion earlier (4th day of incubation) than others and it
showed higher keratinase secretion of 80.75KU. A similar finding was
recorded by Page and Stock [5].
Their experiments also showed that keratinase secretion started early in M.
gypseum on the 3rd day of incubation itself. This might be because of the
presence of perforating organs in M. gypseum which 'facilitates' the
mechanical disruption of keratin and allows the growth of mycelium faster [6].
Though T. rubrum showed its preference for human hair
among the different substrates, the keratinase activity was however much
lower than other organisms. The keratinase activity of T. rubrum showed a
steady increase up to 14 days of incubation. Between 14th and 20th
day, there was a very little increase in enzyme secretion and a pattern of
near to stationary phase was observed. Earlier work has recorded a similar
finding [7].
Current study also showed that the keratinase secretion by T. rubrum was the
least (25.75KU) in chicken feather and moderate (38.07KU) in human nail when
compared to human hair which recorded a peak activity of 45.43KU.
It was also observed that M. gypseum achieved
keratinase secretion of 79.67KU on the 16th day of incubation in
human hair and reaches its maximum activity of 80.75KU on 20th
day of incubation. The results also showed that the secretion pattern of M.
canis was similar to M. gypseum. i.e. M. canis also preferred human hair
than other keratinaceous substrates with peak enzyme activity of 78.72KU.
But the amount of enzyme secretion was relatively lower in M. canis (a
zoophilic dermatophyte) than M. gypseum (a geophilic dermatophyte). The
preference for human hair keratin by M. canis and M. gypseum could be the
reason for severity of infection caused by these organisms on encountering
the human host.
E. floccosum was the only organism in the present
study which showed preference towards human nail with maximum keratinase
activity of 66.28KU which was followed by human hair (56.90KU). This could
be supported by our earlier study [8]
and others [9]
with the isolation of E. floccosum from human nail infections. The
utilization of human hair keratin by E. floccosum was recorded in the
present study. Though, clinical infection of human hair (Tinea captis) by E.
floccosum is not a common phenomenon, the in vitro utilization of human hair
keratin when used as a sole keratin source cannot be ruled out. This current
finding was supported by Cabanes et al. [10]
and Macedo et al. [11].
It was also noted that the severity of infection and
keratinase secretion could be going hand in hand together. The current study
revealed that keratinase secretion of T. rubrum was slow and low compared to
other strains. This in turn denotes that T. rubrum infections would be
passive, chronic and not severe, whereas human infection by M. gypseum or M.
canis would be of severe in nature. Current study supports the above
mentioned fact, because the keratinase enzyme secretion by M. gypseum and M.
canis was very high and very fast compared to T. rubrum. Viani et al. [12,13]
reported that the keratinase secretion was statistically higher in animal
isolates of M.canis isolated from symptomatic dogs and cats. This is
strikingly evident because the isolates of M. canis used in the present
study were of animal origin (dogs). When the keratinase secretion is high
the manifestation of the infection would also be high, in turn the human
immune response to M. gypseum or M. canis infection would also be relatively
higher compared to passive T. rubrum infections. Thus T. rubrum may not get
eliminated from human skin faster, as chronic mild lesions would be mostly
ignored/ untreated by the host. This is the classical feature which
facilitates obligate parasitism and complete anthropization of T. rubrum. We
have reported in our earlier study [14]
that protease moderation in dermatophytes as an attribute to anthropization
and the current study is a resurgence of our hypothesis. Further work is
being carried out to understand the co-relation between the keratinase
digestion products of different substrates by different strains to find out
its link to the molecular structure of the different keratin substrates.
References
1. Kunert, J. Physiology of keratinolytic fungai. In: Kushwaha, R.K.S., Guarro, J. (eds). Revista Iberoamericana de Micologia. Bilbao. Apdo, Bilbao, Spain, 2000 p.77- 85.
2. Duke L, Kaufman G, Ulman Y, Berdicevsky I. The pathogenesis of dermatophyte infections in human skin sections. Journal of Infection. 2004; 48 (2): 175 - 180.
3. Lin X, Chung-Ginn Lee, Casale ES, Shih JCH. Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Applied and Environmental Microbiol. 1992; 58 (10): 3271- 3275.
4. Yamamura S, Yasutaka M, Quamrul H, Ramachandra RS, Yuji M, Kenji Y, Eiichi T. Characterization of a new keratin-degrading bacterium isolated from deer fur. Journal of Bioscience and Bioengineering. 2002; 93 (6): 595- 600.
5. Page WJ, Stock JJ. Phosphate-mediated alteration of the Microsporum gypseum germination protease specificity for substrate: enhanced keratinase activity. J. Bacteriol. 1974; 117 (2): 422- 431.
6. Kanbe, T, Tanaka K. Ultrastructure of the invasion of human hair in-vitro by the keratinophilic fungus Microsporum gypseum. Infection and Immunity. 1982; 38 (2): 706- 715.
7. Apodaca G, McKerrow JH. Regulation of Trichophyton rubrum proteolytic activity. Infection and Immunity. 1989; 57 (10): 3081- 3090.
8. Venkatesan G, Ranjitsingh AJA, Murugesan AG, Gokulshankar S. Trichophyton rubrum - the predominant etiological agent in human dermatophytoses in Chennai, India. African J. Microbiol. Research. May 2007: 009- 012.
9. Erbagci Z, Tuncel A A, Zer Y, Balci I. A prospective epidemiologic survey on the prevalence of onychomycosis and dermatophytosis in male boarding school residents. Mycopathologia. 2005; 159: 347- 352.
10. Cabanes FJ, Abarca L, Bragulat R, Calvo A. Further observations on the keratinolytic activity of strains of the genus Epidermophyton. Mycopatholigia. 1987; 98: 41- 43.
11. Macedo DPC, Neves RPN, Magalhaes OMC, Motta CM DeSouza, Queiroz LAde. Pathogenic aspects of Epidermophyton floccosum langeron et milochevitch as a possible aethiological agent of tinea captis. Braz. J. Microbiol. 2005; 36: 36- 37.
12. Viani FC, Viani PRC, Rivera ING, Goncalves E desilva, Paula CR, Gambale, W. Extracellular proteolytic activity and molecular analysis of Microsporum canis strains isolated from symptomatic and asymptomatic cats. Rev Iberoam Micol. 2007; 24: 19- 23.
13. Viani FC, Dos Santos IJ, Paula CR, Larson CE, Gambale W. Production of extracellular enzymes by Microsporum canis and their role in its virulence. Medical Mycology. 2001; 39 (5): 463 - 468.
14. S Gokul Shankar AJA Ranjitsingh, G Venkatesan, MS Ranjith, GS Vijayalakshmi, M Prabhamanju, S Subashini. Is moderation of protease production an adaptation of well-defined anthropization in dermatophytes? Indian Journal of Pathology and Microbiology. 2010; 53(1): 87- 92. © 2010 Egyptian Dermatology Online Journal |




