|
|
Abstract
Anti-malarial drugs may induce numerous cutaneous
adverse drug reactions as well as exacerbation of psoriasis. Acute
generalized exanthematous pustulosis (AGEP) is a clinical reaction pattern
that is principally drug induced and is characterized by acute, extensive
formation of non-follicular sterile pustules on an erythematous and
oedematous substrate. Hydroxychloroquine (HCQ), an anti-malarial drug widely
used to treat rheumatic and dermatologic diseases, has been described as an
uncommon cause of AGEP.
We report a 45-year-old woman who developed severe
bullous AGEP mimicking toxic epidermal necrolysis after the intake of HCQ.
Introduction
Acute generalized exanthematous pustulosis (AGEP) is a
clinical reaction pattern that is principally drug induced and is
characterized by acute, extensive formation of non-follicular sterile
pustules on an erythematous and oedematous substrate. Hydroxychloroquine (HCQ),
an anti-malarial drug widely used to treat rheumatic and dermatologic
diseases, has been described as an uncommon cause of AGEP.
Case Report
A 45-year-old woman, without personal or familiar
history of psoriasis, was referred to Dermatology for an acute pruriginous
pustular eruption. She had started prednisone 10 mg daily 6 months before
and hydroxychloroquine 400 mg daily 10 days before for sero-negative
polyarthritis under investigation.
The erythematopustular eruption was initially
predominantly on the trunk and proximal limbs, accompanied by intense
pruritus, fever 39°C. The face and mucous membranes were not involved.
Within 5 days, the pustular eruption has rapidly spread to all teguments
realizing an erythroderma with significant oedema (fig.
1). Hydroxychloroquine was immediately
discontinued.
Laboratory studies have
showed an elevated erythrocyte sedimentation rate (46mm/h), marked
leukocytosis 16890 with an elevated neutrophil count (80%). Electrolyte
levels and results of urinalysis, renal and liver function tests were
normal. Skin biopsy revealed subcorneal spongiform pustules, oedema of the
papillary dermis and a mixed inflammatory infiltrate with exocytosis of
neutrophils (fig. 2).
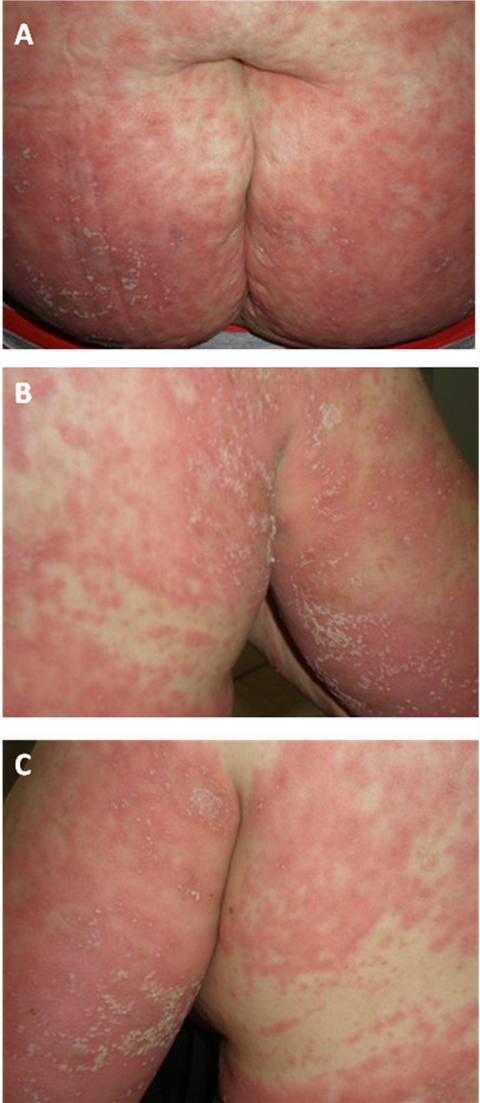 | Fig 1:
Erythemato-pustular eruption; (A) Trunk, (B) and
(C) proximal limbs. |
|
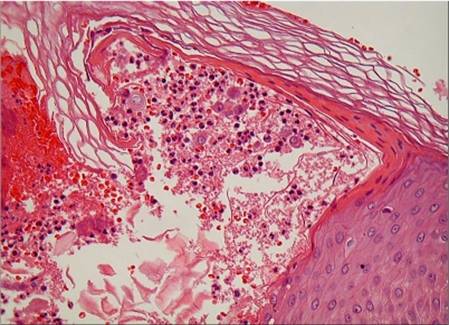 | Fig
2: Histopathological examination showing sub-corneal spongiform
pustules and oedema of the papillary dermis. |
|
After one week of treatment withdrawal, the eruption
continued to progress, involving the face, conjunctival erythema, cheilitis
and alopecia. Large bullae appeared and broke down to form erosions
involving 40% of the total body area mimicking the clinical picture of the
toxic epidermal necrolysis (TEN). The patient was immediately transferred
into the burn specialized service. Within 15 days, the eruption resolved
with re-epithelialisation of erosions and appearance of widespread
post-pustular desquamation.
The Pharmaco-vigilance enquiry has suspected HCQ
(I2B3) because of the latent period between the intake of the culprit and
the appearance of AGEP and the slow resolution of eruption, compatible with
the drug's kinetic elimination.
Discussion
AGEP is an uncommon eruption most often induced by
drugs in than 90% of the cases [1],
by acute infections with enteroviruses, or by mercury [2,3].
It is a rare disease that has been classified as pustular psoriasis of von
Zumbush type for years [4].
In 1968 Backer and Rayan were the first to assume that AGEP represents its
own entity [5].
Subsequently, this disorder was better characterized by Beylot et al. [6]
and Roujeau et al. [7],
who clarified its relationship to pustular psoriasis and assessed the place
of drugs in its aetiology.
HCQ possesses anti-malarial actions and also exerts a
beneficial effect in lupus erythematosus (chronic discoid or systemic) and
acute or chronic rheumatoid arthritis. The precise mechanism of action is
not known. Use of Hydroxychloroquine sulfate in patients with psoriasis may
precipitate a severe attack of psoriasis [8].
Dermatologic reactions to Hydroxychloroquine sulfate
may occur, include bleaching of hair, alopecia, pruritus, skin and mucosal
pigmentation, photosensitivity, and skin eruptions (urticarial,
morbilliform, lichenoid, maculopapular, purpuric, erythema annulare
centrifugum, Stevens-Johnson syndrome, AGEP, and exfoliative dermatitis) [8].
It is important to remember this rare, but severe,
side effect otherwise, in some cases, AGEP may progress into a TEN-like
picture making the diagnosis more difficult [9].
In an important 16 year review of 207 cases of severe
pustular eruptions notified to the French Pharmaco-vigilance Centre [10],
hydroxychloroquine was the third medication associated to AGEP and death
occurred in 4 cases (2%). Because it is essential to discontinue the
causative drug as soon as possible if a pustular eruption occurs, the
notification of side effects by physicians to pharmaco-vigilance centres is
important to public health dissemination of warnings.
AGEP is characterized by acute, extensive formation of
non-follicular sterile pustules on erythematous background, fever, and
peripheral blood leukocytosis. The interval between the administration of
the drug and the onset of the eruption is usually 2 or 3 days for
antibiotics and longer (3-18 days) for drugs other than antibiotics. Our
patient started HCQ 10 days before the eruption [11].
Histologically, AGEP is characterised by subcorneal or
superficial intra-epidermal pustules and a mild spongiform change at the
margins of the pustules. The papillary dermis is usually oedematous, and
perivascular neutrophils or eosinophils infiltrate are shown in the upper
dermis and the presence of necrotic keratinocytes in the epidermis is seen [12].
A standard oral provocation test is a sensitive
diagnostic tool, and it may provide an early confirmatory diagnosis of drug
induced skin eruption, include AGEP. In this case, initial drug dose for an
oral provocation test may be started at 100 mg (half of the therapeutic
dosage). If there are no eruptions after administration of initial drug
dose, an additional dose of 100 mg may be given after an appropriate time
interval [13].
The withdrawal of the responsible drug is the main
treatment for AGEP, in combination with topical corticosteroids and
antipyretics [1,6].
To our knowledge HCQ has been described as an uncommon
cause of AGEP.
Conclusion
This article reports a case of AGEP related to
administration of HCQ. HCQ-induced AGEP is a rare but severe, extensive, and
acute reaction. No specific therapy is available, and correct diagnosis
generally leads to spontaneous resolution once the causative drug has been
withdrawn.
References
1. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute
generalized exanthematous pustulosis (AGEP) - a clinical reaction pattern. J
Cutan Pathol 2001; 28: 113-119
2. Feio AB, Apetato M,
Costa MM, Sa J, Alcantara J. Acute generalized exanthematous pustulosis due
to Coxsackie B4 virus. Acta Med Port 1997; 10: 487- 491
3. Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C et
al. Acute generalized exanthematous pustulosis: analysis of 63 cases. Arch
Dermatol 1991; 127: 1333- 1338
4. Britschgi M,
Steiner UC, Schmid S et al. T-cell involvementin drug-induced acute
generalized exanthematous pustulosis. J Clin Invest 2001; 107: 1433-1441
5. Baker H and Rayan T. Generalized pustular psoriasis. A clinical and
epidemiological study of 104 cases. Br J Dermatol 1968; 80: 771- 793
6. Beylot C, Bioulac P and Doutre MS. Pustuloses exanthématiques aigues
généralisées. A propos de 4 cas. Ann. Dermatol. Venereol 1980; 107: 37- 48
7. Roujeau J, Bioulac-Sage P and Bourseau C. Acute generalized exanthematous
pustulosis: analysis of 63 cases. Arch Dermatol 1991; 127: 1333-1338
8. Plaquenil (hydroxychloroquine sulphate) tablets. Adverse reactions
[Internet]. Bridgewater (NJ): Sanofi; 2006 Oct 5. Available from:
http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/009768s041lbl.pdf.
9. Evans CC, Bergstresser PR. Acute Generalized Exanthematous Pustulosis
precipitated by Hydroxychloroquine. J Am Acad Dermatol 2004; 50(4): 650- 651
10. Saissi EH, Beau-Salinas F, Jonville-Béra AP, Lorette G, Autret-Leca E.
Médicaments Associés à La Survenue d'Une Pustulose Exanthématique Aigue
Généralisée. Ann Dermatol Venereol 2003; 130: 612- 618
11. Machet L, Martin L, Vaillant L. Pustulose exanthématique aigue
généralisée. Ann Dermatol Venereol 2001; 128: 73- 79
12. Burrows NP, Russel Jones RR. Pustular drug eruptions: a
histopathological spectrum. Histopathology 1993; 22: 569- 573
13. Das J, Mandal AC. A study of drug eruptions by provocative tests. Indian
J Dermatol Venereol Leprol 2001; 67: 238- 239©
2010 Egyptian Dermatology Online Journal |


