|
|
Abstract
Background: Cicatricial alopecias are classified into primary
and secondary types according to the initial site of inflammation. In primary
cicatricial alopecias (PCA), the hair follicle is the main target of destruction;
the term secondary cicatricial alopecia implies that follicular destruction
is not the primary pathologic event. Cicatricial alopecia is a trichologic
emergency state which requires a fast and confident confirmation of diagnosis,
as well as aggressive treatment in the active stage of the disease to guard
against permanent destruction of hair follicles therefore trichoscopy may
be applied as a quick and non-invasive method that helps in the differential
diagnosis of diverse diseases leading to cicatricial alopecia.
Objective: To evaluate the potential benefit of trichoscopy in
the clinical diagnosis of primary cicatricial alopecia.
Methods: Trichoscopic examination for 24 patients suffering from
PCA using the DermLite II Pro and 3X optical zoom by Samsung S4 Zoom camera
and their dermoscopic findings were reported.
Results: Our results revealed that among these 24 patients, who
presented with PCA, 7 had lichen planopilaris (LPP), 5 had discoid lupus
erythematosus (DLE), 5 had folliculitis decalvans (FD), 4 had central centrifugal
cicatricial alopecia (CCCA), 2 had dissecting cellulitis (DC) and 1 had
keratosis follicularis spinulosa decalvans (KFSD). The most characteristic
dermoscopic findings in each disease were as follows: perifollicular scales
and peritubular casts in LPP, follicular plugging in DLE, Hair tufting and
pustules in FD, hypotrichosis and white structureless areas in CCCA, diffuse
white area and 3D yellow dots in DC and follicular keratosis in KFSD.
Conclusion: Trichoscopy is a noninvasive tool that significantly
improves the accuracy of the diagnosis of PCA.
Introduction
Cicatricial alopecias are a group of intractable and uncommon hair loss
disorders characterized by permanent hair follicle destruction [1-5]
.The most typical clinical manifestation of cicatricial alopecia is the
loss of visible follicular ostia in a scarring area [4,5].
The histopathological hallmark of a fully developed lesion is the replacement
of the hair follicle structure by fibrous tissue [1,5,6].
Primary cicatricial alopecia (PCA) is a group of disorders, in which the
hair follicle is the main target of destructive inflammation resulting in
irreversible hair loss [4,5,7-9].
PCA were divided into subgroups depending on the predominating inflammatory
infiltrates. Chronic cutaneous lupus erythematosus (CCLE), lichen planopilaris
(LPP), Classic pseudoplade of Brocq (CP), central centrifugal cicatricial
alopecia (CCCA), alopecia mucinosa (AM) and keratosis follicularis spinulosa
decalvans (KFSD) were categorized as ''lymphocytic'' PCA. Frontal fibrosing
alopecia (FFA) and Graham-Little syndrome (GLS) were considered as LLP variants.
The neutrophilic PCA group comprised folliculitis decalvans (FD) and dissecting
cellulitis
⁄
folliculitis (perifolliculitis abscedens et suffodiens) (DC
⁄
DF). Acne keloidalis (AK), acne necrotica (AN) and eruptive
pustular dermatosis (EPD) were classified as ''mixed'' cell infiltrate PCA
[2].
The loss of follicular ostia, which is the most characteristic feature
of PCA, may not be clinically evident in some cases, but could be clearly
visualized under trichoscopy. Indeed, trichoscopy significantly improves
the accuracy of the diagnosis of PCA [4].
Other PCA-associated signs, such as perifollicular erythema or scale, hair
tufting are also detectable [10]. Thus
trichoscopy can helps clinicians assessing PCA disease activity [11].
Patients & Methods
Clinical and dermoscopic examination was performed for 24 patients suffering
from PCA using the DermLite II Pro (3Gen, Inc., San Juan Capistrano, California,
USA.) and 10X optical zoom by Samsung S4 Zoom camera (Samsung Electronics
Co., Ltd., Yeongtong-Gu Suwon-Shi, South Korea) and their dermoscopic findings
were reported.
Results
Among these 24 patients, 7 had LPP, 5 had DLE, 5 had FD, 4 had CCCA,
2 had DC, 1 had KFSD.
Dermoscopic findings in each disease were as follows:
Dermoscopic examination of the LPP patients revealed
perifollicular scales in (85.7%), peritubular casts in 57% of patients,
while white dots, violaceous background, red dots were founded in 42.8%,
28.5%, 28.5% of LPP patients respectively and 14.2% had each of the following
hypotrichosis, diffuse white erythematous areas, broken hairs, thin hairs,
perifollicular hyper pigmentation, white structureless area Figure (1).
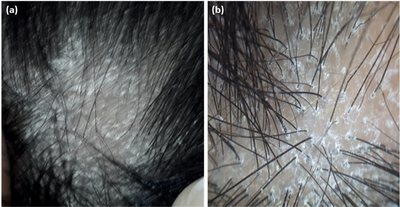
| Fig.1 (a) Clinical image of LPP patient, (b) dermoscopic
images (non contact, polarized, 10x) showing hypotrichosis, perifollicular
scales, peritubular casts and violaceous background. |
|
Five patients of DLE showed follicular plugging in 100% of patients,
arborizing blood vessels in 80%, 40% had each of the following diffuse white
erythematous areas, chrysalis like structures, diffuse scales, red dots,
diffuse erythematous background while 20% had each of hypotrichosis, black
dots, micro ulcers, vellus hair, short cut-off hairs, large yellow dots,
zigzag hair, subcorneal hemorrhage, serpentine and comma- shaped blood vessels,
peripheral brown globules, yellow dots and amicrobial pustulosis Figure
(2).
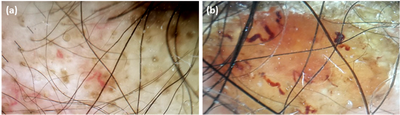 | Fig.2 Dermoscopic images (non contact, polarized) (a- 10x) (b-
30x) of DLE patients shows (a) Hypotrichosis, follicular plugging, arborizing,
serpentine and comma- shaped blood vessels (b) Amicrobial pustulosis. |
|
Hair tufting was present in 100% of FD patients, 80% had each of diffuse
erythematous areas and pustules, while absent follicular ostia, hypotrichosis,
single pig tail like hair, zigzag hair, yellow dots, sub corneal hemorrhage
and dark homogeneous structureless area each were present in 20% of FD patients
Figure (3).
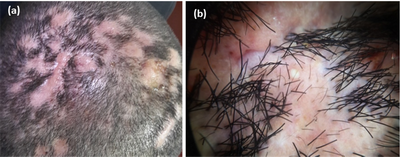 | Fig.3 (a) Clinical image of FD patient, (b) dermoscopic
image (non contact, polarized, 30x) showing hair tufting & pustules. |
|
In CCCA patients 75% had hypotrichosis, 50% had white dots and white
structureless areas, decrease follicular ostia, hair thinning and single
pig tail hair each were present in 25% of CCCA patients Figure (4).
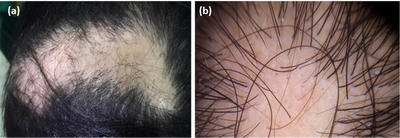 | Fig.4 (a) Clinical image of CCCA patient, (b) dermoscopic
image (non contact, polarized, 30x) showing hypotrichosis, white structureless
areas and decreased follicular ostia. |
|
All the DC patients had diffuse white area, while 50% had each of the
following: hair tufting, subcorneal hemorrhage, follicular plugging, follicular
pustules, linear irregular blood vessels, arborizing blood vessels, black
dots and 3D yellow dots Figure (5).
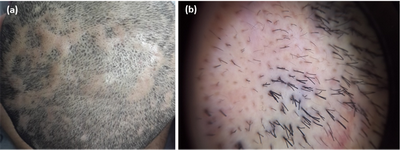 | Fig.5 (a) Clinical image of DC patient, (b) dermoscopic
image (non contact, polarized, 30x) showing White structureless areas &
hair tufting. |
|
The dermoscopic finding of KFSD was black dots, follicular keratosis,
short cut-off hairs, hypotrichosis, perifollicular scales and honeycomb
appearance in addition to short cutoff lashes and follicular scales Figure
(6).
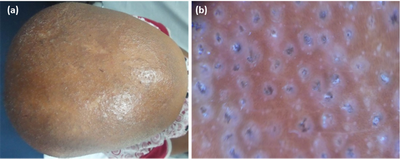 | Fig.6 (a) Clinical image of KFSD patient, (b) dermoscopic
image (non contact, polarized, 30x) showing follicular keratosis, short
cut-off hairs, hypotrichosis & perifollicular scales. |
|
Discussion
Cicatricial alopecia, also called scarring alopecia, represents a "trichologic
emergency" because hair follicles are permanently destroyed so a fast and
confident confirmation of the diagnosis, as well as aggressive treatment
in the case of active disease, is crucial in the management of scarring
alopecia [12]. Trichoscopy may be applied
as a quick and non-invasive auxiliary method in differential diagnosis of
diverse diseases leading to cicatricial alopecia [13].
The category of primary cicatricial alopecia includes a diverse group
of inflammatory diseases of the hair follicles. In PCA, the hair follicle
is the prime target of the destruction as opposed to secondary cicatricial
alopecia, which is caused by a cutaneous, but not specifically folliculocentric,
inflammatory process that eventually encroaches on the follicle and ultimately
destroys it [12].
Primary cicatricial alopecia was classified into 3 main groups: (1) lymphocytic,
(2) neutrophilic, and (3) mixed, based on the nature of the inflammatory
cells observed histologically in and around affected hair follicles [2,14].
Lymphocytic Primary Cicatricial Alopecia including:
Lichen planopilaris:
Lichen planopilaris is the most frequent cause of adult primary scarring
alopecia [15-17].
Three variants of the disease may be distinguished: classic LPP, FFA [18]
Graham Little syndrome[19,20]
.The classic form of LPP are characterized by a violaceous follicular erythema
and perifollicular keratotic lesions [16,19]
.The most characteristic trichoscopic features of LPP are perifollicular
scaling, tubular perifollicular hyperkeratosis, perifollicular inflammation,
violaceous areas [13,21]
and this constant to our result which found that the main dermoscopic finding
in our LPP patients was perifollicular scales in(85.7%), peritubular casts
in 57% and a violaceous background 28.5%, of LPP patients .
Discoid lupus erythematosus:
Discoid lupus erythematosus (DLE) lesion begins as a well-demarcated
round or oval purplish macule or papule and enlarges into an alopecic patch
with follicular plugging, erythema, and adherent scaling. The lesions may
be hypo- or hyperpigmented [22].The most
characteristic trichoscopic features of DLE are keratotic plugs, thick arborizing
vessels, scattered dark-brown discoloration, and blue-gray dots[13,21]
Similar to our result the five patients of DLE showed follicular plugging
while 80% had arborizing blood vessels and 20% had peripheral brown globules.
CCCA:
Central centrifugal cicatricial alopecia (CCCA) is the most common cause
of scarring alopecia among African American women [23].
It is characterized by an area of permanent hair loss that involves the
crown and vertex and spreads centrifugally over time. Miteva and Tosti in
2014, stated that there were no published studies on the dermatoscopic features
of CCCA [24], so they retrospectively reviewed
the dermatoscopic images of 51 women with pathologically confirmed diagnoses
of CCCA [25] and their results revealed
that the most common dermoscopic finding of CCCA were Honeycomb pigmented
network, terminal hairs, vellus hairs, peripilar white halo, pin-point white
dots, white patches, erythema, scales, asterisk-like brown blotches, broken
hairs and dark peripilar halo. Also our results revealed that 75% of our
CCCA patients had hypotrichosis and 50% had white dots and white structureless
areas.
Keratosis Follicularis Spinulosa Decalvans
This inherited condition causes follicular keratotic papules and pustules
producing progressive cicatricial alopecia. Dermoscopic features are very
similar to those of LPP, showing decreased hair density with loss of follicular
openings, hyperkeratotic perifollicular white scales, perifollicular erythema,
and occasionally perifollicular pustules [26].
We had only 1 patient with KFSD who had most of this dermoscopic finding
(follicular keratosis, short cut off hairs, hypotrichosis and perifollicular
scales).
Neutrophilic Primary Cicatricial Alopecia including:
Folliculitis Decalvans:
The disease predominantly involves the vertex and occipital area of the
scalp. The hallmark of folliculitis decalvans is the presence of multiple
hairs emerging from one single dilated follicular opening, other signs included
recurrent follicular pustules, erythema, dark yellow-gray scales, follicular
hyperkeratosis, erosions, and hemorrhagic crusts, most prominent around
the follicles. In the course of the disease, small to extensive irregularly
shaped patches of cicatricial alopecia develop [3,16,27,28].
Trichoscopy of folliculitis decalvans shows tufted hairs, perifollicular
hyperplasia [13], yellowish tubular scaling
and follicular pustules, white and milky red areas lacking follicular openings
[13,21].
In constant to this, hair tufting was present in 100% of our FD patients,
and 80% had each of diffuse erythematous areas and pustules, while absent
follicular ostia, hypotrichosis, and dark homogeneous structureless area
each were present in 20% of FD patients.
Dissecting cellulitis
Is a chronic, progressive, inflammatory disease that occurs most commonly
in young adults [17]. The disease usually
starts with occlusion of follicular openings on the scalp vertex or occiput.
Later, perifollicular pustules, nodules, and abscesses with interconnecting
sinus tracts develop. Nodules are firm or fluctuant and contain purulent
material, tufted hairs may be present [27,29-31].
In dissecting cellulitis, trichoscopy shows yellow structureless areas and
3D yellow dots imposed over dystrophic hair shafts. Black dots, pinpoint-like
vessels with a whitish halo occasionally are present [21].
End-stage fibrotic lesions are characterized by confluent ivory-white or
white areas lacking follicular openings [13,21].
All our DC patients had diffuse white area and subcorneal hemorrhage, while
50% had each of the following hair tufting, follicular plugging, follicular
pustules, linear irregular blood vessels, arborizing blood vessels, black
dots and 3D yellow dots.
Conclusion
Trichoscopy could be applied as a quick and non-invasive method that
help the early diagnosis of different types of cicatricial alopecia allowing
rapid as well as aggressive treatment in the case of active disease in order
to slow down the progression of the condition.
References
1. Whiting DA. Cicatricial alopecia: Clinico-pathological
findings and treatment. Clin Dermatol. 2001; 19: 211-225.
2. Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro
J, Sinclair R, Solomon A, Sperling L, Stenn K, Whiting DA, Bernardo O, Bettencourt
M, Bolduc C, Callendar V, Elston D, Hickman J, Ioffreda M, King L, Linzon
C, McMichael A, Miller J, Mulinari F, Trancik R. Summary of North American
Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia,
Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol.
2003; 48: 103-110.
3. Mirmirani P, Willey A, Headington JT, Stenn K, McCalmont
TH, Price VH. Primary cicatricial alopecia: histopathologic findings do
not distinguish clinical variants. J Am Acad Dermatol. 2005; 52: 637-643.
4. Harries MJ, Sinclair RD, Macdonald-Hull S, Whiting DA,
Griffiths CE, Paus R. Management of primary cicatricial alopecias: options
for treatment. Br J Dermatol. 2008; 159: 1-22.
5. Harries MJ, Trueb RM, Tosti A, Messenger AG, Chaudhry
I, Whiting DA, Sinclair R, Griffiths CE, Paus R. How not to get scar(r)ed:
pointers to the correct diagnosis in patients with suspected primary cicatricial
alopecia. Br J Dermatol. 2009; 160: 482-501.
6. Sperling LC. Scarring alopecia and the dermatopathologist.
J Cutan Pathol. 2001; 28: 333-342.
7. Somani N, Bergfeld WF. Cicatricial alopecia: classification
and histopathology. Dermatol Ther. 2008; 21: 221-237.
8. Harries MJ, Meyer KC, Paus R. Hair loss as a result of
cutaneous autoimmunity: frontiers in the immunopathogenesis of primary cicatricial
alopecia. Autoimmun Rev. 2009; 8: 478-483.
9. Harries MJ, Paus R. The pathogenesis of primary cicatricial
alopecias. Am J Pathol. 2010; 177: 2152-2162.
10. Inui S. Trichoscopy for common hair loss diseases:
algorithmic method for diagnosis. J Dermatol. 2011; 38: 71-75.
11. Tosti A, Torres F, Misciali C, et al. Follicular red
dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus.
Arch Dermatol. 2009; 145: 1406-1409.
12. Otberg N, Wu WY, McElwee KJ, Shapiro J. Diagnosis and
Management of Primary Cicatricial Alopecia: Part I. SKINmed: Dermatology
for the Clinician. 2008:7:19-26.
13. Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik
O, Czuwara J, Olszewska M, Rudnicka L Trichoscopy of cicatricial alopecia.
Journal of Drugs in Dermatology. 2012, 11:753-758.
14. Olsen E, Stenn K, Bergfeld W, Cotsarelis G, Price V,
Shapiro J, Sinclair R, Solomon A, Sperling L, Whiting D. Update on cicatricial
alopecia. J Investig Dermatol Symp Proc. 2003;8:18-19.
15. Ochoa BE, King Jr LE, Price VH. Lichen planopilaris:
annual incidence in four hair referral centers in the United States. J Am
Acad Dermatol. 2008;58:352-3.
16. Otberg N, Kang H, Alzolibani AA, Shapiro J. Folliculitis
decalvans. Dermatol Ther. 2008;21:238-44.
17. Price V, Mirmirani P, editors. Cicatricial alopecia:
an approach to diagnosis and management. New York: Springer; 2011.
18. Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia
presenting with components of Piccardi-Lassueur-Graham-Little syndrome.J
Am Acad Dermatol. 2007;57:15-8.
19. Assouly P, Reygagne P. Lichen planopilaris: update
on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
20. Wiseman MC, Shapiro J. Scarring alopecia. J Cutan Med
Surg. 1999;3 :45-8.
21. Rudnicka L, Olszewska M, Rakowska A. Trichoscopy update
.2011.J Dermatol Case Rep 2011;5:82-8. 3.
22. Walling HW, Sontheimer RD. Cutaneous lupus erythematosus:
issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6): 365-81.
23. Olsen EA, Callender V, Sperling L, McMichael A, Anstrom
KJ, Bergfeld W, Durden F, Roberts J, Shapiro J, Whiting DA. Central scalp
alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-7.
24. Miteva M, Tosti A. Dermatoscopic features of central
centrifugal cicatricial alopecia. J Am Acad Dermatol. 2014; 71:443-9.
25. Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am
Acad Dermatol. 2012;67:1040-8
26. Tostia A, Torresb F. Dermoscopy in the Diagnosis of
Hair and Scalp Disorders. Actas Dermosifiliogr. 2009; 100:114-9.
27. Wu WY, Otberg N, McElwee KJ, Shapiro J. Diagnosis and
management of primary cicatricial alopecia: part II. Skinmed. 2008;7:78-83.
28. Stefanato CM. Histopathology of alopecia: a clinicopathological
approach to diagnosis. Histopathology. 2010;56:24-38.
29. Lugovic Mihic L, Tomas D, Situm M, Krolo I, Sebetic
K, Sjerobabski-Masnec I. Perifolliculitis capitis abscedens et suffodiens
in a caucasian: diagnostic and therapeutic challenge. Acta Dermatovenerol
Croat. 2011;19:98-102.
30. Branisteanu DE, Molodoi A, Ciobanu D, Badescu A, Stoica
LE. The importance of histopathologic aspects in the diagnosis of dissecting
cellulitis of the scalp. Rom J Morphol Embryol. 2009;50:719-24.
31. Tchernev G. Folliculitis et perifolliculitis capitis
abscedens et suffodiens controlled with a combination therapy: systemic
antibiosis (metronidazole plus clindamycin), dermatosurgical approach, and
high-dose isotretinoin. Indian J Dermatol .2011;56:318-20.
© 2015 Egyptian Dermatology Online Journal
|






